Conventional chemotherapy- and radiotherapy-induced cancer senescence, which is characterized by poor proliferation, drug resistance, and senescence-associated secretory phenotype, has gained attention as contributing to cancer relapse and the development of an immunosuppressive tumor microenvironment. However, the association between cancer senescence and anti-tumor immunity is not fully understood. Here, we demonstrate that senescent cancer cells increase the level of PD-L1 by promoting its transcription and glycosylation. We identify ribophorin 1 as a key regulator of PD-L1 glycosylation during cancer senescence. Ribophorin 1 depletion reduces this elevated level of PD-L1 through the ER-lysosome-associated degradation pathway, thereby increasing the susceptibility of senescent cancer cells to T-cell-mediated killing. Consistently, ribophorin 1 depletion suppresses tumor growth by decreasing PD-L1 levels and boosting cytotoxic T lymphocyte activity in male mice. Moreover, ribophorin 1-targeted or anti-PD-1 therapy reduces the number of senescent cancer cells in irradiated tumors and suppresses cancer recurrence through the activation of cytotoxic T lymphocytes. These results provide crucial insights into how senescent cancer cells can escape T-cell immunity following cancer treatment and thereby contribute to cancer recurrence. Our findings also highlight the therapeutic promise of targeting senescent cancer cells for cancer treatment.
© 2025. The Author(s).
Product Citations: 26
In Nature Communications on 3 January 2025 by Hwang, H. J., Kang, D., et al.
-
Mus musculus (House mouse)
-
Cancer Research
Thymocytes trigger self-antigen-controlling pathways in immature medullary thymic epithelial stages.
In eLife on 21 February 2022 by Lopes, N., Boucherit, N., et al.
Interactions of developing T cells with Aire+ medullary thymic epithelial cells expressing high levels of MHCII molecules (mTEChi) are critical for the induction of central tolerance in the thymus. In turn, thymocytes regulate the cellularity of Aire+ mTEChi. However, it remains unknown whether thymocytes control the precursors of Aire+ mTEChi that are contained in mTEClo cells or other mTEClo subsets that have recently been delineated by single-cell transcriptomic analyses. Here, using three distinct transgenic mouse models, in which antigen presentation between mTECs and CD4+ thymocytes is perturbed, we show by high-throughput RNA-seq that self-reactive CD4+ thymocytes induce key transcriptional regulators in mTEClo and control the composition of mTEClo subsets, including Aire+ mTEChi precursors, post-Aire and tuft-like mTECs. Furthermore, these interactions upregulate the expression of tissue-restricted self-antigens, cytokines, chemokines, and adhesion molecules important for T-cell development. This gene activation program induced in mTEClo is combined with a global increase of the active H3K4me3 histone mark. Finally, we demonstrate that these self-reactive interactions between CD4+ thymocytes and mTECs critically prevent multiorgan autoimmunity. Our genome-wide study thus reveals that self-reactive CD4+ thymocytes control multiple unsuspected facets from immature stages of mTECs, which determines their heterogeneity.
© 2022, Lopes et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Expression of the Phosphatase Ppef2 Controls Survival and Function of CD8+ Dendritic Cells.
In Frontiers in Immunology on 28 February 2019 by Zwick, M., Ulas, T., et al.
Apoptotic cell death of Dendritic cells (DCs) is critical for immune homeostasis. Although intrinsic mechanisms controlling DC death have not been fully characterized up to now, experimentally enforced inhibition of DC-death causes various autoimmune diseases in model systems. We have generated mice deficient for Protein Phosphatase with EF-Hands 2 (Ppef2), which is selectively expressed in CD8+ DCs, but not in other related DC subtypes such as tissue CD103+ DCs. Ppef2 is down-regulated rapidly upon maturation of DCs by toll-like receptor stimuli, but not upon triggering of CD40. Ppef2-deficient CD8+ DCs accumulate the pro-apoptotic Bcl-2-like protein 11 (Bim) and show increased apoptosis and reduced competitve repopulation capacities. Furthermore, Ppef2-/- CD8+ DCs have strongly diminished antigen presentation capacities in vivo, as CD8+ T cells primed by Ppef2-/- CD8+ DCs undergo reduced expansion. In conclusion, our data suggests that Ppef2 is crucial to support survival of immature CD8+ DCs, while Ppef2 down-regulation during DC-maturation limits T cell responses.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 29 June 2018 by Huang, W., Luo, J., et al.
T cell homeostatic proliferation (HP) is regulated by T cell receptor (TCR) signals and homeostatic cytokines, and suggested to be proportional to TCR signal strength. However, we show here that ITK, a positive regulator of TCR signaling, negatively tunes CD8 + T cell HP, metabolism, and effector function. Under lymphopenic environments, Itk −/− CD8 + T cells exhibit significant increase in T cell-intrinsic HP, which requires mTOR activity and can be driven by T cell-T cell interaction. TCR signals through ITK tune IL-7-mediated CD8 + T cell metabolism and HP in a mTOR-dependent manner. The lack of ITK also resulted in enhanced effector cell fate programming, antigen sensitivity and anti-tumor immunity by HP cells. Thus, TCR signaling via ITK, is a negative tuner of CD8 + T cell homeostasis, metabolism and effector function, and may be a target for clinical benefit in cancer therapy. h4>One Sentence Summary/h4> TCR signal strength had been long-thought to be proportional to T cell proliferation and effector function, here we demonstrate a counterintuitive role of the TCR signaling through ITK in negatively tuning proliferation under lymphopenic conditions via regulating mTOR activity, T cell metabolism, proliferation, and effector function.
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Effector CD4+ T cells recognize intravascular antigen presented by patrolling monocytes.
In Nature Communications on 21 February 2018 by Westhorpe, C. L. V., Norman, M. U., et al.
Although effector CD4+ T cells readily respond to antigen outside the vasculature, how they respond to intravascular antigens is unknown. Here we show the process of intravascular antigen recognition using intravital multiphoton microscopy of glomeruli. CD4+ T cells undergo intravascular migration within uninflamed glomeruli. Similarly, while MHCII is not expressed by intrinsic glomerular cells, intravascular MHCII-expressing immune cells patrol glomerular capillaries, interacting with CD4+ T cells. Following intravascular deposition of antigen in glomeruli, effector CD4+ T-cell responses, including NFAT1 nuclear translocation and decreased migration, are consistent with antigen recognition. Of the MHCII+ immune cells adherent in glomerular capillaries, only monocytes are retained for prolonged durations. These cells can also induce T-cell proliferation in vitro. Moreover, monocyte depletion reduces CD4+ T-cell-dependent glomerular inflammation. These findings indicate that MHCII+ monocytes patrolling the glomerular microvasculature can present intravascular antigen to CD4+ T cells within glomerular capillaries, leading to antigen-dependent inflammation.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
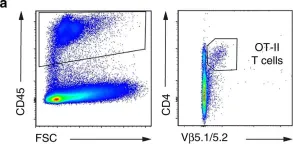
In Nat Commun on 21 February 2018 by Westhorpe, C. L. V., Norman, M. U., et al.
Fig.5.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1