To increase antibody affinity against pathogens, positively selected GC-B cells initiate cell division in the light zone (LZ) of germinal centers (GCs). Among these, higher-affinity clones migrate to the dark zone (DZ) and vigorously proliferate by utilizing energy provided by oxidative phosphorylation (OXPHOS). However, it remains unknown how positively selected GC-B cells adapt their metabolism for cell division in the glycolysis-dominant, cell cycle arrest-inducing, hypoxic LZ microenvironment. Here, we show that microRNA (miR)-155 mediates metabolic reprogramming during positive selection to protect high-affinity clones. Mechanistically, miR-155 regulates H3K36me2 levels in hypoxic conditions by directly repressing the histone lysine demethylase, Kdm2a, whose expression increases in response to hypoxia. The miR-155-Kdm2a interaction is crucial for enhancing OXPHOS through optimizing the expression of vital nuclear mitochondrial genes under hypoxia, thereby preventing excessive production of reactive oxygen species and subsequent apoptosis. Thus, miR-155-mediated epigenetic regulation promotes mitochondrial fitness in high-affinity GC-B cells, ensuring their expansion and consequently affinity maturation.
© 2024. The Author(s).
Product Citations: 45
Epi-microRNA mediated metabolic reprogramming counteracts hypoxia to preserve affinity maturation.
In Nature Communications on 3 December 2024 by Nakagawa, R., Llorian, M., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
Metabolic fitness of IgA+ plasma cells in the gut requires DOCK8.
In Mucosal Immunology on 1 June 2024 by Zhang, B., Chen, S., et al.
Dedicator of cytokinesis 8 (DOCK8) mutations lead to a primary immunodeficiency associated with recurrent gastrointestinal infections and poor antibody responses but, paradoxically, heightened IgE to food antigens, suggesting that DOCK8 is central to immune homeostasis in the gut. Using Dock8-deficient mice, we found that DOCK8 was necessary for mucosal IgA production to multiple T cell-dependent antigens, including peanut and cholera toxin. Yet DOCK8 was not necessary in T cells for this phenotype. Instead, B cell-intrinsic DOCK8 was required for maintenance of antigen-specific IgA-secreting plasma cells (PCs) in the gut lamina propria. Unexpectedly, DOCK8 was not required for early B cell activation, migration, or IgA class switching. An unbiased interactome screen revealed novel protein partners involved in metabolism and apoptosis. Dock8-deficient IgA+ B cells had impaired cellular respiration and failed to engage glycolysis appropriately. These results demonstrate that maintenance of the IgA+ PC compartment requires DOCK8 and suggest that gut IgA+ PCs have unique metabolic requirements for long-term survival in the lamina propria.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
In Cell Reports on 20 December 2022 by Osma-García, I. C., Capitan-Sobrino, D., et al.
B cell lymphopoiesis requires dynamic modulation of the B cell transcriptome for timely coordination of somatic mutagenesis and DNA repair in progenitor B (pro-B) cells. Here, we show that, in pro-B cells, the RNA-binding proteins T cell intracellular antigen 1 (TIA1) and TIA1-like protein (TIAL1) act redundantly to enable developmental progression. They are global splicing regulators that control the expression of hundreds of mRNAs, including those involved in DNA damage repair. Mechanistically, TIA1 and TIAL1 bind to 5' splice sites for exon definition, splicing, and expression of DNA damage sensors, such as Chek2 and Rif1. In their absence, pro-B cells show exacerbated DNA damage, altered P53 expression, and increased cell death. Our study uncovers the importance of tight regulation of RNA splicing by TIA1 and TIAL1 for the expression of integrative transcriptional programs that control DNA damage sensing and repair during B cell development.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Genetics
-
Immunology and Microbiology
In Nature Immunology on 1 December 2022 by Lee, R. D., Knutson, T. P., et al.
The nuclear corepressors NCOR1 and NCOR2 interact with transcription factors involved in B cell development and potentially link these factors to alterations in chromatin structure and gene expression. Herein, we demonstrate that Ncor1/2 deletion limits B cell differentiation via impaired recombination, attenuates pre-BCR signaling and enhances STAT5-dependent transcription. Furthermore, NCOR1/2-deficient B cells exhibited derepression of EZH2-repressed gene modules, including the p53 pathway. These alterations resulted in aberrant Rag1 and Rag2 expression and accessibility. Whole-genome sequencing of Ncor1/2 DKO B cells identified increased number of structural variants with cryptic recombination signal sequences. Finally, deletion of Ncor1 alleles in mice facilitated leukemic transformation, whereas human leukemias with less NCOR1 correlated with worse survival. NCOR1/2 mutations in human leukemia correlated with increased RAG expression and number of structural variants. These studies illuminate how the corepressors NCOR1/2 regulate B cell differentiation and provide insights into how NCOR1/2 mutations may promote B cell transformation.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In STAR Protocols on 17 June 2022 by Desikan, S. A., Chavan, S., et al.
Highly enriched germinal center (GC) B cell populations are essential for studying humoral immunity. Current MACS protocols that isolate untouched GC B cells require GC induction and typically require further FACS purification with direct antibody labeling to achieve sufficiently high purities. We present a MACS protocol with progressive and repeated negative selections that yields highly purified untouched GC B cells from both unimmunized and GC-induced mice and allows further FACS isolation of unlabeled GC B cells from remaining debris by scatter.
© 2022 The Author(s).
-
Immunology and Microbiology
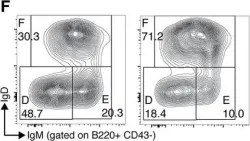
In Front Immunol on 7 April 2018 by Liu, R., King, A., et al.
Fig.1.F

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1