Despite major therapeutic advances in the treatment of acute lymphoblastic leukemia (ALL), resistances and long-term toxicities still pose significant challenges. Cyclins and their associated cyclin-dependent kinases are one focus of cancer research when looking for targeted therapies. We discovered cyclin C to be a key factor for B-cell ALL (B-ALL) development and maintenance. While cyclin C is not essential for normal hematopoiesis, CcncΔ/Δ BCR::ABL1+ B-ALL cells fail to elicit leukemia in mice. RNA sequencing experiments revealed a p53 pathway deregulation in CcncΔ/Δ BCR::ABL1+ cells resulting in the inability of the leukemic cells to adequately respond to stress. A genome-wide CRISPR/Cas9 loss-of-function screen supplemented with additional knock-outs unveiled a dependency of human B-lymphoid cell lines on CCNC. High cyclin C levels in B-cell precursor (BCP) ALL patients were associated with poor event-free survival and increased risk of early disease recurrence after remission. Our findings highlight cyclin C as a potential therapeutic target for B-ALL, particularly to enhance cancer cell sensitivity to stress and chemotherapy.
Product Citations: 65
In Haematologica on 1 April 2025 by Trifinopoulos, J., List, J., et al.
-
Mus musculus (House mouse)
-
Cancer Research
-
Cardiovascular biology
-
Immunology and Microbiology
Increased peritoneal B1-like cells during acute phase of human septic peritonitis.
In IScience on 19 July 2024 by von Loeffelholz, C., Winkler, R., et al.
Sepsis is a life-threatening condition caused by dysregulated host responses to infection. Myeloid cell accumulation and lymphocyte decline are widely recognized phenomena in septic patients. However, the fate of specific immune cells remains unclear. Here, we report the results of a human explorative study of patients with septic peritonitis and patients undergoing abdominal surgery without sepsis. We analyzed pairwise peritoneal fluid and peripheral blood taken 24 h after surgery to characterize immediate immune cell changes. Our results show that myeloid cell expansion and lymphocyte loss occur in all patients undergoing open abdominal surgery, indicating that these changes are not specific to sepsis. However, B1-like lymphocytes were specifically increased in the peritoneal fluid of septic patients, correlating positively with sequential organ failure assessment (SOFA) and acute physiology and chronic health evaluation II (APACHE-II) clinical severity scores. In support of this notion, we identified an accumulation of peritoneal B1b lymphocytes in septic mice.
© 2024 The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
Molecular basis for differential Igk versus Igh V(D)J joining mechanisms.
In Nature on 1 June 2024 by Zhang, Y., Li, X., et al.
In developing B cells, V(D)J recombination assembles exons encoding IgH and Igκ variable regions from hundreds of gene segments clustered across Igh and Igk loci. V, D and J gene segments are flanked by conserved recombination signal sequences (RSSs) that target RAG endonuclease1. RAG orchestrates Igh V(D)J recombination upon capturing a JH-RSS within the JH-RSS-based recombination centre1-3 (RC). JH-RSS orientation programmes RAG to scan upstream D- and VH-containing chromatin that is presented in a linear manner by cohesin-mediated loop extrusion4-7. During Igh scanning, RAG robustly utilizes only D-RSSs or VH-RSSs in convergent (deletional) orientation with JH-RSSs4-7. However, for Vκ-to-Jκ joining, RAG utilizes Vκ-RSSs from deletional- and inversional-oriented clusters8, inconsistent with linear scanning2. Here we characterize the Vκ-to-Jκ joining mechanism. Igk undergoes robust primary and secondary rearrangements9,10, which confounds scanning assays. We therefore engineered cells to undergo only primary Vκ-to-Jκ rearrangements and found that RAG scanning from the primary Jκ-RC terminates just 8 kb upstream within the CTCF-site-based Sis element11. Whereas Sis and the Jκ-RC barely interacted with the Vκ locus, the CTCF-site-based Cer element12 4 kb upstream of Sis interacted with various loop extrusion impediments across the locus. Similar to VH locus inversion7, DJH inversion abrogated VH-to-DJH joining; yet Vκ locus or Jκ inversion allowed robust Vκ-to-Jκ joining. Together, these experiments implicated loop extrusion in bringing Vκ segments near Cer for short-range diffusion-mediated capture by RC-based RAG. To identify key mechanistic elements for diffusional V(D)J recombination in Igk versus Igh, we assayed Vκ-to-JH and D-to-Jκ rearrangements in hybrid Igh-Igk loci generated by targeted chromosomal translocations, and pinpointed remarkably strong Vκ and Jκ RSSs. Indeed, RSS replacements in hybrid or normal Igk and Igh loci confirmed the ability of Igk-RSSs to promote robust diffusional joining compared with Igh-RSSs. We propose that Igk evolved strong RSSs to mediate diffusional Vκ-to-Jκ joining, whereas Igh evolved weaker RSSs requisite for modulating VH joining by RAG-scanning impediments.
© 2024. The Author(s).
-
Mus musculus (House mouse)
Three-dimensional chromatin reorganization regulates B cell development during ageing.
In Nature Cell Biology on 1 June 2024 by Ma, F., Cao, Y., et al.
The contribution of three-dimensional genome organization to physiological ageing is not well known. Here we show that large-scale chromatin reorganization distinguishes young and old bone marrow progenitor (pro-) B cells. These changes result in increased interactions at the compartment level and reduced interactions within topologically associated domains (TADs). The gene encoding Ebf1, a key B cell regulator, switches from compartment A to B with age. Genetically reducing Ebf1 recapitulates some features of old pro-B cells. TADs that are most reduced with age contain genes important for B cell development, including the immunoglobulin heavy chain (Igh) locus. Weaker intra-TAD interactions at Igh correlate with altered variable (V), diversity (D) and joining (J) gene recombination. Our observations implicate three-dimensional chromatin reorganization as a major driver of pro-B cell phenotypes that impair B lymphopoiesis with age.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
-
Cell Biology
-
Immunology and Microbiology
Extra centrosomes delay DNA damage-driven tumorigenesis.
In Science Advances on 29 March 2024 by Braun, V. Z., Karbon, G., et al.
Deregulated centrosome numbers are frequently found in human cancer and can promote malignancies in model organisms. Current research aims to clarify if extra centrosomes are cause or consequence of malignant transformation, and if their biogenesis can be targeted for therapy. Here, we show that oncogene-driven blood cancer is inert to genetic manipulation of centrosome numbers, whereas the formation of DNA damage-induced malignancies is delayed. We provide first evidence that this unexpected phenomenon is connected to extra centrosomes eliciting a pro-death signal engaging the apoptotic machinery. Apoptosis induction requires the PIDDosome multi-protein complex, as it can be abrogated by loss of any of its three components, Caspase-2, Raidd/Cradd, or Pidd1. BCL2 overexpression equally blocks cell death, documenting for the first time induction of mitochondrial apoptosis downstream of extra centrosomes. Our findings demonstrate context-dependent effects of centrosome amplification during transformation and ask to adjust current belief that extra centrosomes are intrinsically pro-tumorigenic.
-
Mus musculus (House mouse)
-
Cell Biology
-
Genetics
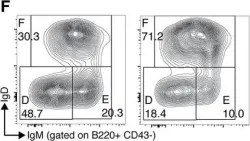
In Front Immunol on 7 April 2018 by Liu, R., King, A., et al.
Fig.1.F

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1