The T-lineage restricted protein THEMIS has been shown to play a critical role in T cell development. THEMIS, via its distinctive CABIT domains, inhibits the catalytic activity of the tyrosine phosphatase SHP1 (PTPN6). SHP1 and THEMIS bind to the ubiquitous cytosolic adapter GRB2, and the purported formation of a tri-molecular THEMIS-GRB2-SHP1 complex facilitates inactivation of SHP1 by THEMIS. The importance of this function of GRB2 among its numerous documented activities is unclear as GRB2 binds to multiple proteins and participates in several signaling responses in thymocytes. Here, we show that similar to Themis-/- thymocytes, the primary molecular defect in GRB2-deficient thymocytes is increased catalytically active SHP1 and the developmental block in GRB2-deficient thymocytes is alleviated by deletion or inhibition of SHP1 and is exacerbated by SHP1 overexpression. Thus, the principal role of GRB2 during T cell development is to promote THEMIS-mediated inactivation of SHP1 thereby enhancing the sensitivity of TCR signaling in CD4+CD8+ thymocytes to low affinity positively selecting self-ligands.
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Product Citations: 24
GRB2 promotes thymocyte positive selection by facilitating THEMIS-mediated inactivation of SHP1.
In The Journal of Experimental Medicine on 3 July 2023 by Choi, S., Hatzihristidis, T., et al.
-
Mus musculus (House mouse)
In eLife on 27 January 2023 by Tsyklauri, O., Chadimova, T., et al.
Regulatory T cells (Tregs) are indispensable for maintaining self-tolerance by suppressing conventional T cells. On the other hand, Tregs promote tumor growth by inhibiting anticancer immunity. In this study, we identified that Tregs increase the quorum of self-reactive CD8+ T cells required for the induction of experimental autoimmune diabetes in mice. Their major suppression mechanism is limiting available IL-2, an essential T-cell cytokine. Specifically, Tregs inhibit the formation of a previously uncharacterized subset of antigen-stimulated KLRK1+ IL-7R+ (KILR) CD8+ effector T cells, which are distinct from conventional effector CD8+ T cells. KILR CD8+ T cells show superior cell-killing abilities in vivo. The administration of agonistic IL-2 immunocomplexes phenocopies the absence of Tregs, i.e., it induces KILR CD8+ T cells, promotes autoimmunity, and enhances antitumor responses in mice. Counterparts of KILR CD8+ T cells were found in the human blood, revealing them as a potential target for immunotherapy.
© 2023, Tsyklauri et al.
-
FC/FACS
-
Immunology and Microbiology
In ImmunoHorizons on 11 October 2022 by Crooks, S. D., Varga, S. M., et al.
Influenza virus-specific tissue-resident memory CD8 T cells (Trms) targeting conserved viral proteins provide strain-transcending heterosubtypic immunity to infection. Trms in the lung combat reinfection through rapid cytolytic function and production of inflammatory cytokines to recruit other immune cells. Influenza-specific Trms are also generated in the lung draining mediastinal lymph node (mLN) and can provide immunity to heterologous virus infection in this tissue, although their role in combating influenza infection is less well defined. Functional avidity, a measure of T cell sensitivity to Ag stimulation, correlates with control of viral infection and may be important for immune detection of recently infected cells, when low numbers of surface peptide-MHC complexes are displayed. However, the functional avidity of influenza-specific Trms has not been previously compared with that of other memory CD8 T cell subsets. In this article, a methodology is presented to compare the functional avidity of CD8 T cell subsets across murine tissues, with a focus on influenza-specific mLNs compared with splenic CD8 T cells, by stimulating both populations in the same well to account for CD8 T cell-extrinsic variables. The functional avidity of influenza-specific mLN effector CD8 T cells is slightly increased relative to splenic effector CD8 T cells. However, CD103+ mLN Trms display increased functional avidity compared with splenic memory CD8 T cells and CD103- memory CD8 T cells within the mLN. In contrast, lung-derived CD103+ Trms did not exhibit enhanced functional avidity. mLN CD103+ Trms also exhibit increased TCR expression, providing a potential mechanism for their enhanced functional avidity.
Copyright © 2022 The Authors.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 12 November 2021 by Tsyklauri, O., Chadimova, T., et al.
Regulatory T cells (Tregs) are indispensable for maintaining self-tolerance by suppressing conventional T cells. On the other hand, Tregs may promote tumor growth by inhibiting anti-cancer immunity. In this study, we identified that Tregs increase the quorum of self-reactive CD8 + T cells required for the induction of experimental autoimmune diabetes. Their major suppression mechanism is limiting available IL-2, an essential T-cell cytokine. Specifically, Tregs inhibit the formation of a previously uncharacterized subset of antigen-stimulated KLRK1 + IL7R + (KILR) CD8 + effector T cells, which are distinct from conventional effector CD8 + T cells. KILR CD8 + T cells show a superior cell killing abilities in vivo. The administration of agonistic IL-2 immunocomplexes phenocopies the absence of Tregs, i.e., it induces KILR CD8 + T cells, promotes autoimmunity, and enhances anti-tumor responses. Counterparts of KILR CD8 + T cells were found in the human blood, revealing them as a potential target for immunotherapy.
-
Immunology and Microbiology
Nociceptive sensory neurons promote CD8 T cell responses to HSV-1 infection.
In Nature Communications on 18 May 2021 by Filtjens, J., Roger, A., et al.
Host protection against cutaneous herpes simplex virus 1 (HSV-1) infection relies on the induction of a robust adaptive immune response. Here, we show that Nav1.8+ sensory neurons, which are involved in pain perception, control the magnitude of CD8 T cell priming and expansion in HSV-1-infected mice. The ablation of Nav1.8-expressing sensory neurons is associated with extensive skin lesions characterized by enhanced inflammatory cytokine and chemokine production. Mechanistically, Nav1.8+ sensory neurons are required for the downregulation of neutrophil infiltration in the skin after viral clearance to limit the severity of tissue damage and restore skin homeostasis, as well as for eliciting robust CD8 T cell priming in skin-draining lymph nodes by controlling dendritic cell responses. Collectively, our data reveal an important role for the sensory nervous system in regulating both innate and adaptive immune responses to viral infection, thereby opening up possibilities for new therapeutic strategies.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
-
Neuroscience
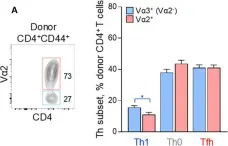
In Front Immunol on 29 June 2018 by Danelli, L., Donnarumma, T., et al.
Fig.4.A

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1