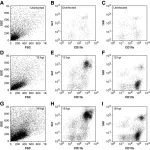
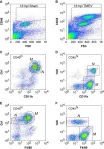
Myocarditis is a life-threatening inflammatory disease, but lacks effective treatment options. Hydroxychloroquine (HCQ), an established antimalarial agent, is used widely to manage rheumatic disorders. This research aimed to evaluate the efficacy of HCQ in treating myocarditis.
A mouse model of experimental autoimmune myocarditis (EAM) was used to evaluate the therapeutic effects of HCQ on cardiac function, inflammation and fibrosis. Echocardiography, histology and cytokine assays were performed to assess cardiac function and inflammatory responses. Single-cell RNA sequencing was employed to analyse immune cell populations and chemotactic activity. C-X-C motif chemokine ligand 16 (CXCL16) levels were measured in cardiac tissue and serum, while YY1 expression was measured by western blotting in macrophages and cardiac tissue. Flow cytometry was used to evaluate immune cell infiltration and migration.
HCQ improved cardiac function in acute and chronic myocarditis. HCQ treatment reduced inflammation, fibrosis and immune cell infiltration in myocarditis models. Single-cell RNA sequencing revealed that HCQ lowered inflammatory cell proportions and suppressed macrophage chemotaxis. HCQ reduced YY1 levels, leading to the down-regulation of CXCL16 expression in macrophages and inhibition of CXCL16-mediated chemotaxis to Th17 and natural killer T (NKT) cells. CXCL16 neutralizing antibodies improved cardiac function and reduced inflammation in myocarditis.
HCQ improves cardiac function and reduces inflammation in myocarditis by inhibiting CXCL16 expression in macrophages, by suppressing its transcription factor YY1, which in turn reduced the chemotaxis of Th17 and NKT cells. HCQ is a promising therapeutic agent for myocarditis.
© 2025 British Pharmacological Society.
Product Citations: 250
In British Journal of Pharmacology on 1 August 2025 by Xuan, Y., Gao, X., et al.
-
Immunology and Microbiology
-
Pharmacology
In vivo haemopoietic stem cell gene therapy enabled by postnatal trafficking.
In Nature on 28 May 2025 by Milani, M., Fabiano, A., et al.
Lentiviral vector (LV)-mediated ex vivo gene therapy for haematopoietic stem and progenitor cells (HSPCs) has delivered on the promise of a 'one-and-done' treatment for several genetic diseases1. However, ex vivo manipulation and patient conditioning before transplantation are major hurdles that could be overcome by an in vivo approach. Here we demonstrate that in vivo gene delivery to HSPCs after systemic LV administration is enabled by the substantial trafficking of these cells from the liver to the bone marrow in newborn mice. We improved gene-transfer efficiency using a phagocytosis-shielded LV, successfully reaching bona fide HSPCs capable of long-term multilineage output and engraftment after serial transplantation, as confirmed by clonal tracking. HSPC mobilization further increased gene transfer, extending the window of intervention, although permissiveness to LV transduction declined with age. We successfully tested this in vivo strategy in mouse models of adenosine deaminase deficiency, autosomal recessive osteopetrosis and Fanconi anaemia. Interestingly, in vivo gene transfer provided a selective advantage to corrected HSPCs in Fanconi anaemia, leading to near-complete haematopoietic reconstitution and prevention of bone marrow failure. Given that circulating HSPCs in humans are also most abundant shortly after birth, in vivo HSPC gene transfer holds strong translational potential across multiple diseases.
© 2025. The Author(s).
-
Stem Cells and Developmental Biology
In Molecular Neurodegeneration on 5 March 2025 by Sirerol-Piquer, M. S., Perez-Villalba, A., et al.
Cytoplasmic alpha-synuclein (αSyn) aggregates are a typical feature of Parkinson's disease (PD). Extracellular insoluble αSyn can induce pathology in healthy neurons suggesting that PD neurodegeneration may spread through cell-to-cell transfer of αSyn proteopathic seeds. Early pro-homeostatic reaction of microglia to toxic forms of αSyn remains elusive, which is especially relevant considering the recently uncovered microglial molecular diversity. Here, we show that periventricular microglia of the subependymal neurogenic niche monitor the cerebrospinal fluid and can rapidly phagocytize and degrade different aggregated forms of αSyn delivered into the lateral ventricle. However, this clearing ability worsens with age, leading to an increase in microglia with aggregates in aged treated mice, an accumulation also observed in human PD samples. We also show that exposure of aged microglia to aggregated αSyn isolated from human PD samples results in the phosphorylation of the endogenous protein and the generation of αSyn seeds that can transmit the pathology to healthy neurons. Our data indicate that while microglial phagocytosis rapidly clears toxic αSyn, aged microglia can contribute to synucleinopathy spreading.
© 2025. The Author(s).
-
FC/FACS
-
Neuroscience
In Journal of Inflammation Research on 24 February 2025 by Jang, J. H., Song, Y., et al.
Persistent inflammation resulting from injury, infection, or arthritis contributes to both peripheral and central sensitization. Various combinations of natural extracts have been explored to minimize the side effects associated with conventional medications. Shinbaro, which has traditionally been used in Eastern medicine to treat inflammatory conditions, was chosen due to its known anti-inflammatory properties. However, previous studies have not yet investigated the combined administration of celecoxib and Shinbaro for their anti-inflammatory and analgesic effects. In this study, we examined the anti-inflammatory and analgesic effects of combining celecoxib with Shinbaro in a complete Freund's adjuvant (CFA)-induced inflammatory pain model.
We randomly assigned 66 mice to 6 groups (n = 11 per group) and administered intraplantar injections of 100 μL CFA or saline into their right hind paw, followed by oral administration of Shinbaro (100 mg/kg), celecoxib (15 or 30 mg/kg), or both 30 minutes later. Behavioral assessments were conducted blindly at baseline and on days 1, 3, and 7 post-injection. The right hind paw and spinal cord were harvested 3 days post-injection to examine the molecular mechanisms, including macrophage infiltration in the right hind paw, as well as glial cell activation and inflammatory cytokine levels in the spinal cord. Statistical analysis was performed using Tukey's post-hoc test.
The combination of Shinbaro (100 mg/kg) and celecoxib (15 mg/kg) synergistically reduced mechanical hyperalgesia and paw edema by preventing the conversion of monocytes to macrophages and inhibiting macrophage infiltration. Moreover, it decreased the expression of pro-inflammatory cytokines and mediators in the spinal cord by inhibiting spinal microglial activation.
The combination of Shinbaro and celecoxib demonstrates significant anti-inflammatory and analgesic effects, suggesting its potential for managing inflammatory pain with fewer side effects than conventional therapies.
© 2025 Jang et al.
-
Immunology and Microbiology
In Nature Communications on 7 February 2025 by Kwon, J., Kawase, H., et al.
Macrophages express numerous G protein-coupled receptors (GPCRs) that regulate adhesion, migration, and activation, but the function of orphan receptor GPRC5B in macrophages is unknown. Both resident peritoneal and bone marrow-derived macrophages from myeloid-specific GPRC5B-deficient mice show increased migration and phagocytosis, resulting in improved bacterial clearance in a peritonitis model. In other models such as myocardial infarction, increased myeloid cell recruitment has adverse effects. Mechanistically, we found that GPRC5B physically interacts with GPCRs of the prostanoid receptor family, resulting in enhanced signaling through the prostaglandin E receptor 2 (EP2). In GPRC5B-deficient macrophages, EP2-mediated anti-inflammatory effects are diminished, resulting in hyperactivity. Using in silico modelling and docking, we identify residues potentially mediating GPRC5B/EP2 dimerization and show that their mutation results in loss of GPRC5B-mediated facilitation of EP2 signaling. Finally, we demonstrate that decoy peptides mimicking the interacting sequence are able to reduce GPRC5B-mediated facilitation of EP2-induced cAMP signaling in macrophages.
© 2025. The Author(s).
-
Immunology and Microbiology
In Cancer Cell on 13 May 2019 by Segovia, M., Russo, S., et al.
Fig.2.F

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Cancer Cell by CiteAb, provided under a CC-BY license
Image 1 of 7
In Nat Commun on 24 June 2015 by Singh, N. K., Kotla, S., et al.
Fig.7.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 7
In Nat Commun on 24 June 2015 by Singh, N. K., Kotla, S., et al.
Fig.7.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 7
In J Neuroinflammation on 9 March 2012 by Howe, C. L., Lafrance-Corey, R. G., et al.
Fig.9.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from J Neuroinflammation by CiteAb, provided under a CC-BY license
Image 1 of 7
In J Neuroinflammation on 9 March 2012 by Howe, C. L., Lafrance-Corey, R. G., et al.
Fig.5.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from J Neuroinflammation by CiteAb, provided under a CC-BY license
Image 1 of 7
In J Neuroinflammation on 9 March 2012 by Howe, C. L., Lafrance-Corey, R. G., et al.
Fig.7.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from J Neuroinflammation by CiteAb, provided under a CC-BY license
Image 1 of 7
In J Neuroinflammation on 9 March 2012 by Howe, C. L., Lafrance-Corey, R. G., et al.
Fig.2.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from J Neuroinflammation by CiteAb, provided under a CC-BY license
Image 1 of 7