There is active crosstalk between tumor cells and the tumor microenvironment during metastatic progression, a process that is significantly affected by obesity, particularly in breast cancer. Here we analyze the impact of a high fat diet (HFD) on metastasis, focusing on the role of platelets in the formation of premetastatic niches (PMNs). We find that a HFD provokes pre-activation of platelets and endothelial cells, promoting the formation of PMNs in the lung. These niches are characterized by increased vascular leakiness, platelet activation and overexpression of fibronectin in both platelets and endothelial cells. A HFD promotes interactions between platelets, tumor cells and endothelial cells within PMNs, enhancing tumor cell homing and metastasis. Importantly, therapeutic interventions like anti-platelet antibody administration or a dietary switch reduce metastatic cell homing and outgrowth. Moreover, blocking fibronectin reduces the interaction of tumor cells with endothelial cells. Importantly, when coagulation parameters prior to neoadjuvant treatment are considered, triple negative breast cancer (TNBC) female patients with reduced Partial Thromboplastin time (aPTT) had a significantly shorter time to relapse. These findings highlight how diet and platelet activation in pre-metastatic niches affect tumor cell homing and metastasis, suggesting potential therapeutic interventions and prognostic markers for TNBC patients.
© 2025. The Author(s).
Product Citations: 40
The impact of a high fat diet and platelet activation on pre-metastatic niche formation.
In Nature Communications on 2 April 2025 by Hergueta-Redondo, M., Sánchez-Redondo, S., et al.
Talin1 dysfunction is genetically linked to systemic capillary leak syndrome.
In JCI Insight on 20 December 2024 by Elefant, N., Rouni, G., et al.
Systemic capillary leak syndrome (SCLS) is a rare life-threatening disorder due to profound vascular leak. The trigger and the cause of the disease are currently unknown and there is no specific treatment. Here, we identified a rare heterozygous splice-site variant in the TLN1 gene in a familial SCLS case, suggestive of autosomal dominant inheritance with incomplete penetrance. Talin1 has a key role in cell adhesion by activating and linking integrins to the actin cytoskeleton. This variant causes in-frame skipping of exon 54 and is predicted to affect talin's C-terminal actin-binding site (ABS3). Modeling the SCLS-TLN1 variant in TLN1-heterozygous endothelial cells (ECs) disturbed the endothelial barrier function. Similarly, mimicking the predicted actin-binding disruption in TLN1-heterozygous ECs resulted in disorganized endothelial adherens junctions. Mechanistically, we established that the SCLS-TLN1 variant, through the disruption of talin's ABS3, sequestrates talin's interacting partner, vinculin, at cell-extracellular matrix adhesions, leading to destabilization of the endothelial barrier. We propose that pathogenic variants in TLN1 underlie SCLS, providing insight into the molecular mechanism of the disease that can be explored for future therapeutic interventions.
-
Mus musculus (House mouse)
Preprint on BioRxiv : the Preprint Server for Biology on 24 September 2024 by Sajib, M. S., Zahra, F. T., et al.
Abstract/Summary The endothelial barrier plays an active role in transendothelial tumor cell migration during metastasis, however, the endothelial regulatory elements of this step remain obscure. Here we show that endothelial RhoA activation is a determining factor during this process. Breast tumor cell-induced endothelial RhoA activation is the combined outcome of paracrine IL-8-dependent and cell-to-cell contact β 1 integrin-mediated mechanisms, with elements of this pathway correlating with clinical data. Endothelial-specific RhoA blockade or in vivo deficiency inhibited the transendothelial migration and metastatic potential of human breast tumor and three murine syngeneic tumor cell lines, similar to the pharmacological blockade of the downstream RhoA pathway. These findings highlight endothelial RhoA as a potent, universal target in the tumor microenvironment for anti-metastatic treatment of solid tumors.
-
Cancer Research
In The Journal of Clinical Investigation on 16 July 2024 by Mathis, T., Baudin, F., et al.
Neovascular age-related macular degeneration (nAMD) remains a major cause of visual impairment and puts considerable burden on patients and health care systems. l-DOPA-treated Parkinson's disease (PD) patients have been shown to be partially protected from nAMD, but the mechanism remains unknown. Using murine models that combine 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced (MPTP-induced) PD and laser-induced nAMD with standard PD treatment of l-DOPA/DOPA-decarboxylase inhibitor or specific dopamine receptor inhibitors, we here demonstrate that l-DOPA treatment-induced increase of dopamine-mediated dopamine receptor D2 (DRD2) signaling inhibits choroidal neovascularization independently of MPTP-associated nigrostriatal pathway lesion. Analyzing a retrospective cohort of more than 200,000 patients with nAMD receiving anti-VEGF treatment from the French nationwide insurance database, we show that DRD2 agonist-treated PD patients have a significantly delayed age of onset of nAMD and reduced need for anti-VEGF therapies, similar to the effects of the l-DOPA treatment. While providing a mechanistic explanation for an intriguing epidemiological observation, our findings suggest that systemic DRD2 agonists might constitute an adjuvant therapy to delay and reduce the need for anti-VEGF therapy in patients with nAMD.
-
Mus musculus (House mouse)
-
Neuroscience
Comparative In Vivo Imaging of Retinal Structures in Tree Shrews, Humans, and Mice.
In ENeuro on 1 March 2024 by Grannonico, M., Miller, D. A., et al.
Rodent models, such as mice and rats, are commonly used to examine retinal ganglion cell damage in eye diseases. However, as nocturnal animals, rodent retinal structures differ from primates, imposing significant limitations in studying retinal pathology. Tree shrews (Tupaia belangeri) are small, diurnal paraprimates that exhibit superior visual acuity and color vision compared with mice. Like humans, tree shrews have a dense retinal nerve fiber layer (RNFL) and a thick ganglion cell layer (GCL), making them a valuable model for investigating optic neuropathies. In this study, we applied high-resolution visible-light optical coherence tomography to characterize the tree shrew retinal structure in vivo and compare it with that of humans and mice. We quantitatively characterize the tree shrew's retinal layer structure in vivo, specifically examining the sublayer structures within the inner plexiform layer (IPL) for the first time. Next, we conducted a comparative analysis of retinal layer structures among tree shrews, mice, and humans. We then validated our in vivo findings in the tree shrew inner retina using ex vivo confocal microscopy. The in vivo and ex vivo analyses of the shrew retina build the foundation for future work to accurately track and quantify the retinal structural changes in the IPL, GCL, and RNFL during the development and progression of human optic diseases.
Copyright © 2024 Grannonico et al.
-
Mus musculus (House mouse)
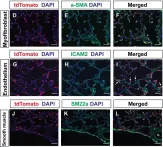
In Elife on 4 September 2018 by Li, R., Bernau, K., et al.
Fig.2.D

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 1