Pleomorphic rhabdomyosarcoma (PRMS) predominantly arises in adult skeletal musculature and is usually associated with poor prognosis. Thus, effective treatments must be developed. PRMS is a rare tumor; therefore, it is critical to develop an experimental system to understand the cellular and molecular mechanisms of PRMS. We previously demonstrated that PRMS develops after p53 gene deletion and oncogenic K-Ras expression in the skeletal muscle tissue. In that study, oncogenic K-Ras-expressing cells were diverse and the period until disease onset was difficult to control. In this study, we developed an experimental system to address this problem. Single cell-derived murine cell lines, designated as RMS310 and RMSg2, were established by limiting the dilution of cells from a lung metastatic tumor colony that were positive for various cancer stem cells and activated skeletal muscle-resident stem/progenitor cell marker genes by RT-PCR. All cell lines stably recapitulated the histological characteristics of human PRMS as bizarre giant cells, desmin-positive cells, and lung metastases in C57BL/6 mice. All subclones of the RMSg2 cells by the limiting dilution in vitro could seed PRMS subcutaneously, and as few as 500 RMSg2 cells were sufficient to form tumors. These results suggest that the RMSg2 cells are multipotent cancer cells that partially combine the properties of skeletal muscle-resident stem/progenitor cells and high tumorigenicity. Thus, our model system's capacity to regenerate tumor tissue in vivo and maintain stable cells in vitro makes it useful for developing therapeutics to treat PRMS.
Product Citations: 43
In Experimental Animals / Japanese Association for Laboratory Animal Science on 9 November 2023 by Saito, H. & Suzuki, N.
-
Mus musculus (House mouse)
-
Cancer Research
-
Veterinary Research
Spatial heterogeneity of bone marrow endothelial cells unveils a distinct subtype in the epiphysis.
In Nature Cell Biology on 1 October 2023 by Iga, T., Kobayashi, H., et al.
Bone marrow endothelial cells (BMECs) play a key role in bone formation and haematopoiesis. Although recent studies uncovered the cellular taxonomy of stromal compartments in the bone marrow (BM), the complexity of BMECs is not fully characterized. In the present study, using single-cell RNA sequencing, we defined a spatial heterogeneity of BMECs and identified a capillary subtype, termed type S (secondary ossification) endothelial cells (ECs), exclusively existing in the epiphysis. Type S ECs possessed unique phenotypic characteristics in terms of structure, plasticity and gene expression profiles. Genetic experiments showed that type S ECs atypically contributed to the acquisition of bone strength by secreting type I collagen, the most abundant bone matrix component. Moreover, these cells formed a distinct reservoir for haematopoietic stem cells. These findings provide the landscape for the cellular architecture in the BM vasculature and underscore the importance of epiphyseal ECs during bone and haematopoietic development.
© 2023. The Author(s).
-
Mus musculus (House mouse)
-
Cell Biology
In Cell Stem Cell on 1 December 2022 by Porpiglia, E., Mai, T., et al.
In aging, skeletal muscle strength and regenerative capacity decline, due in part to functional impairment of muscle stem cells (MuSCs), yet the underlying mechanisms remain elusive. Here, we capitalize on mass cytometry to identify high CD47 expression as a hallmark of dysfunctional MuSCs (CD47hi) with impaired regenerative capacity that predominate with aging. The prevalent CD47hi MuSC subset suppresses the residual functional CD47lo MuSC subset through a paracrine signaling loop, leading to impaired proliferation. We uncover that elevated CD47 levels on aged MuSCs result from increased U1 snRNA expression, which disrupts alternative polyadenylation. The deficit in aged MuSC function in regeneration can be overcome either by morpholino-mediated blockade of CD47 alternative polyadenylation or antibody blockade of thrombospondin-1/CD47 signaling, leading to improved regeneration in aged mice, with therapeutic implications. Our findings highlight a previously unrecognized age-dependent alteration in CD47 levels and function in MuSCs, which underlies reduced muscle repair in aging.Copyright © 2022 Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Stem Cells and Developmental Biology
In JCI Insight on 3 December 2020 by Lu, S., Jolly, A. J., et al.
Resident vascular adventitial SCA1+ progenitor (AdvSca1) cells are essential in vascular development and injury. However, the heterogeneity of AdvSca1 cells presents a unique challenge in understanding signaling pathways orchestrating their behavior in homeostasis and injury responses. Using smooth muscle cell (SMC) lineage-tracing models, we identified a subpopulation of AdvSca1 cells (AdvSca1-SM) originating from mature SMCs that undergo reprogramming in situ and exhibit a multipotent phenotype. Here we employed lineage tracing and RNA-sequencing to define the signaling pathways regulating SMC-to-AdvSca1-SM cell reprogramming and AdvSca1-SM progenitor cell phenotype. Unbiased hierarchical clustering revealed that genes related to hedgehog/WNT/beta-catenin signaling were significantly enriched in AdvSca1-SM cells, emphasizing the importance of this signaling axis in the reprogramming event. Leveraging AdvSca1-SM-specific expression of GLI-Kruppel family member GLI1 (Gli1), we generated Gli1-CreERT2-ROSA26-YFP reporter mice to selectively track AdvSca1-SM cells. We demonstrated that physiologically relevant vascular injury or AdvSca1-SM cell-specific Kruppel-like factor 4 (Klf4) depletion facilitated the proliferation and differentiation of AdvSca1-SM cells to a profibrotic myofibroblast phenotype rather than macrophages. Surprisingly, AdvSca1-SM cells selectively contributed to adventitial remodeling and fibrosis but little to neointima formation. Together, these findings strongly support therapeutics aimed at preserving the AdvSca1-SM cell phenotype as a viable antifibrotic approach.
-
IHC-IF
Smooth Muscle Cell Reprogramming in Aortic Aneurysms.
In Cell Stem Cell on 2 April 2020 by Chen, P. Y., Qin, L., et al.
The etiology of aortic aneurysms is poorly understood, but it is associated with atherosclerosis, hypercholesterolemia, and abnormal transforming growth factor β (TGF-β) signaling in smooth muscle. Here, we investigated the interactions between these different factors in aortic aneurysm development and identified a key role for smooth muscle cell (SMC) reprogramming into a mesenchymal stem cell (MSC)-like state. SMC-specific ablation of TGF-β signaling in Apoe-/- mice on a hypercholesterolemic diet led to development of aortic aneurysms exhibiting all the features of human disease, which was associated with transdifferentiation of a subset of contractile SMCs into an MSC-like intermediate state that generated osteoblasts, chondrocytes, adipocytes, and macrophages. This combination of medial SMC loss with marked increases in non-SMC aortic cell mass induced exuberant growth and dilation of the aorta, calcification and ossification of the aortic wall, and inflammation, resulting in aneurysm development.
Copyright © 2020 Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Stem Cells and Developmental Biology
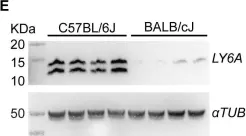
In PLoS One on 15 November 2019 by Huang, Q., Chan, K. Y., et al.
Fig.2.E

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1