Hematopoietic stem cells (HSCs) produce all essential cellular components of the blood. Stromal cell lines supporting HSCs follow a vascular smooth muscle cell (vSMC) differentiation pathway, suggesting that some hematopoiesis-supporting cells originate from vSMC precursors. These pericyte-like precursors were recently identified in the aorta-gonad-mesonephros (AGM) region; however, their role in the hematopoietic development in vivo remains unknown. Here, we identify a subpopulation of NG2+Runx1+ perivascular cells that display a sclerotome-derived vSMC transcriptomic profile. We show that deleting Runx1 in NG2+ cells impairs the hematopoietic development in vivo and causes transcriptional changes in pericytes/vSMCs, endothelial cells and hematopoietic cells in the murine AGM. Importantly, this deletion leads also to a significant reduction of HSC reconstitution potential in the bone marrow in vivo. This defect is developmental, as NG2+Runx1+ cells were not detected in the adult bone marrow, demonstrating the existence of a specialised pericyte population in the HSC-generating niche, unique to the embryo.
© 2024. The Author(s).
Product Citations: 92
In Nature Communications on 23 February 2024 by Gonzalez Galofre, Z. N., Kilpatrick, A. M., et al.
-
Mus musculus (House mouse)
Preprint on BioRxiv : the Preprint Server for Biology on 17 November 2022 by Ikami, K., Shoffner, S., et al.
During mouse gametogenesis, germ cells derived from the same progenitor are connected via intercellular bridges forming germline cysts, within which asymmetrical or symmetrical cell fate occurs in female and male germ cells respectively. Here, we have identified branched cyst structures in mice, and investigated their formation and function in oocyte determination. In fetal female cysts, 16.8% of the germ cells are connected by three or four bridges, namely branching germ cells. These germ cells are preferentially protected from cell death and cyst fragmentation, and to accumulate organelles and cytoplasm from sister germ cells to become primary oocytes. Changes in cyst structure and single-cell mRNA profiles suggested that cytoplasmic transport in germline cysts is conducted in a directional manner, in which cellular content is first transported locally between peripheral germ cells and further enriched in branching germ cells, a process causing selective germ cell loss in cysts. Cyst fragmentation occurs extensively in female cysts, but not in male cysts. Male cysts in fetal and adult testes have branched cyst structures, without differential cell fates between germ cells. During cyst formation, E-cadherin junctions between germ cells position intercellular bridges to form branched cysts. Disrupted junction formation in E-cadherin-depleted cysts led to an altered ratio in branched cysts. Germ cell-specific E-cadherin knockout resulted in reductions in primary oocyte number and oocyte size. These findings shed light on the mechanism of how the size of the ovarian reserve, the number of primary oocytes available to sustain adult ovarian function, is determined during ovary formation.
-
Mus musculus (House mouse)
PDGFRβ+ cells play a dual role as hematopoietic precursors and niche cells during mouse ontogeny.
In Cell Reports on 19 July 2022 by Sá da Bandeira, D., Kilpatrick, A. M., et al.
Hematopoietic stem cell (HSC) generation in the aorta-gonad-mesonephros region requires HSC specification signals from the surrounding microenvironment. In zebrafish, PDGF-B/PDGFRβ signaling controls hematopoietic stem/progenitor cell (HSPC) generation and is required in the HSC specification niche. Little is known about murine HSPC specification in vivo and whether PDGF-B/PDGFRβ is involved. Here, we show that PDGFRβ is expressed in distinct perivascular stromal cell layers surrounding the mid-gestation dorsal aorta, and its deletion impairs hematopoiesis. We demonstrate that PDGFRβ+ cells play a dual role in murine hematopoiesis. They act in the aortic niche to support HSPCs, and in addition, PDGFRβ+ embryonic precursors give rise to a subset of HSPCs that persist into adulthood. These findings provide crucial information for the controlled production of HSPCs in vitro.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
In Science Advances on 4 March 2022 by Ma, L., Tang, Q., et al.
Ten-eleven translocation (Tet) enzymes promote DNA demethylation by oxidizing 5-methylcytosine. They are expressed during development and are essential for mouse gastrulation. However, their postgastrulation functions are not well established. We find that global or endothelial-specific loss of all three Tet enzymes immediately after gastrulation leads to reduced number of hematopoietic stem and progenitor cells (HSPCs) and lethality in mid-gestation mouse embryos. This is due to defects in specification of HSPCs from endothelial cells (ECs) that compromise primitive and definitive hematopoiesis. Mechanistically, loss of Tet enzymes in ECs led to hypermethylation and down-regulation of NFκB1 and master hematopoietic transcription factors (Gata1/2, Runx1, and Gfi1b). Restoring Tet catalytic activity or overexpression of these factors in Tet-deficient ECs rescued hematopoiesis defects. This establishes Tet enzymes as activators of hematopoiesis programs in ECs for specification of HSPCs during embryogenesis, which is distinct from their roles in adult hematopoiesis, with implications in deriving HSPCs from pluripotent cells.
-
Mus musculus (House mouse)
-
Genetics
Conditionally pathogenic genetic variants of a hematopoietic disease-suppressing enhancer.
In Science Advances on 10 December 2021 by Soukup, A. A., Matson, D. R., et al.
Human genetic variants are classified on the basis of potential pathogenicity to guide clinical decisions. However, mechanistic uncertainties often preclude definitive categorization. Germline coding and enhancer variants within the hematopoietic regulator GATA2 create a bone marrow failure and leukemia predisposition. The conserved murine enhancer promotes hematopoietic stem cell (HSC) genesis, and a single-nucleotide human variant in an Ets motif attenuates chemotherapy-induced hematopoietic regeneration. We describe “conditionally pathogenic” (CP) enhancer motif variants that differentially affect hematopoietic development and regeneration. The Ets motif variant functioned autonomously in hematopoietic cells to disrupt hematopoiesis. Because an epigenetically silenced normal allele can exacerbate phenotypes of a pathogenic heterozygous variant, we engineered a bone marrow failure model harboring the Ets motif variant and a severe enhancer mutation on the second allele. Despite normal developmental hematopoiesis, regeneration in response to chemotherapy, inflammation, and a therapeutic HSC mobilizer was compromised. The CP paradigm informs mechanisms underlying phenotypic plasticity and clinical genetics.
-
Mus musculus (House mouse)
-
Genetics
In Development on 1 May 2015 by Ikami, K., Tokue, M., et al.
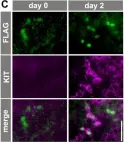
Fig.5.C

-
IF
-
Mus musculus (House mouse)
Collected and cropped from Development by CiteAb, provided under a CC-BY license
Image 1 of 4
In Development on 1 May 2015 by Ikami, K., Tokue, M., et al.
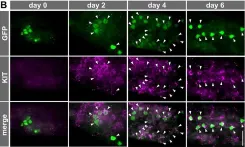
Fig.5.E

-
IF
-
Mus musculus (House mouse)
Collected and cropped from Development by CiteAb, provided under a CC-BY license
Image 1 of 4
In Development on 1 May 2015 by Ikami, K., Tokue, M., et al.
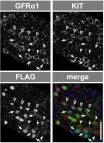
Fig.2.B

-
IF
-
Mus musculus (House mouse)
Collected and cropped from Development by CiteAb, provided under a CC-BY license
Image 1 of 4
In Development on 1 May 2015 by Ikami, K., Tokue, M., et al.
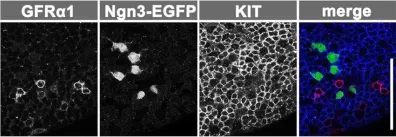
Fig.1.C

-
IF
-
Mus musculus (House mouse)
Collected and cropped from Development by CiteAb, provided under a CC-BY license
Image 1 of 4