Intestinal barrier dysfunction is a prevalent and varied manifestation of acute pancreatitis (AP). Molecular mechanisms underlying the early intestinal barrier in AP remain poorly understood.
To explore the biological processes and mechanisms of intestinal injury associated with AP, and to find potential targets for early prevention or treatment of intestinal barrier injury.
This study utilized single-cell RNA sequencing of the small intestine, alongside in vitro and in vivo experiments, to examine intestinal barrier function homeostasis during the early stages of AP and explore involved biological processes and potential mechanisms.
Seventeen major cell types and 33232 cells were identified across all samples, including normal, AP1 (4x caerulein injections, animals sacrificed 2 h after the last injection), and AP2 (8x caerulein injections, animals sacrificed 4 h after the last injection). An average of 980 genes per cell was found in the normal intestine, compared to 927 in the AP1 intestine and 1382 in the AP2 intestine. B cells, dendritic cells, mast cells (MCs), and monocytes in AP1 and AP2 showed reduced numbers compared to the normal intestine. Enterocytes, brush cells, enteroendocrine cells, and goblet cells maintained numbers similar to the normal intestine, while cytotoxic T cells and natural killer (NK) cells increased. Enterocytes in early AP exhibited elevated programmed cell death and intestinal barrier dysfunction but retained absorption capabilities. Cytotoxic T cells and NK cells showed enhanced pathogen-fighting abilities. Activated MCs, secreted chemokine (C-C motif) ligand 5 (CCL5), promoted neutrophil and macrophage infiltration and contributed to barrier dysfunction.
These findings enrich our understanding of biological processes and mechanisms in AP-associated intestinal injury, suggesting that CCL5 from MCs is a potential target for addressing dysfunction.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Product Citations: 182
In World Journal of Gastroenterology : WJG on 28 March 2025 by Wei, Z. X., Jiang, S. H., et al.
In-depth functional analysis of BRD9 in fetal hematopoiesis reveals context-dependent roles.
In IScience on 21 March 2025 by Zhang, Y., Nomura, M., et al.
The hierarchical organization of hematopoietic stem cells (HSCs) governing adult hematopoiesis has been extensively investigated. However, the dynamic epigenomic transition from fetal to adult hematopoiesis remains incompletely understood, particularly regarding the involvement of epigenetic factors. In this study, we investigate the roles of BRD9, an essential component of the non-canonical BAF (ncBAF) complex known to govern the fate of adult HSCs, in fetal hematopoiesis. Consistent with observations in adult hematopoiesis, BRD9 loss impairs fetal HSC stemness and disturbs erythroid maturation. Intriguingly, the impact on myeloid lineage was discrepant: BRD9 loss inhibited and promoted myeloid differentiation in fetal and adult models, respectively. Through comprehensive transcriptomic and epigenomic analysis, we elucidate the differential roles of BRD9 in a context- and lineage-dependent manner. Our data uncover how BRD9/ncBAF complex modulates transcription in a stage-specific manner, providing deeper insights into the epigenetic regulation underlying the transition from fetal to adult hematopoiesis.
© 2025 The Authors.
Multiscale footprints reveal the organization of cis-regulatory elements.
In Nature on 1 February 2025 by Hu, Y., Horlbeck, M. A., et al.
Cis-regulatory elements (CREs) control gene expression and are dynamic in their structure and function, reflecting changes in the composition of diverse effector proteins over time1. However, methods for measuring the organization of effector proteins at CREs across the genome are limited, hampering efforts to connect CRE structure to their function in cell fate and disease. Here we developed PRINT, a computational method that identifies footprints of DNA-protein interactions from bulk and single-cell chromatin accessibility data across multiple scales of protein size. Using these multiscale footprints, we created the seq2PRINT framework, which uses deep learning to allow precise inference of transcription factor and nucleosome binding and interprets regulatory logic at CREs. Applying seq2PRINT to single-cell chromatin accessibility data from human bone marrow, we observe sequential establishment and widening of CREs centred on pioneer factors across haematopoiesis. We further discover age-associated alterations in the structure of CREs in murine haematopoietic stem cells, including widespread reduction of nucleosome footprints and gain of de novo identified Ets composite motifs. Collectively, we establish a method for obtaining rich insights into DNA-binding protein dynamics from chromatin accessibility data, and reveal the architecture of regulatory elements across differentiation and ageing.
© 2025. The Author(s).
-
Mus musculus (House mouse)
In The Journal of Clinical Investigation on 21 January 2025 by Cui, X., Hou, L., et al.
The bone marrow (BM) niche is critical in regulating hematopoiesis, and sexual dimorphism and its underlying mechanism in the BM niche and its impact on hematopoiesis are not well understood. We show that male mice exhibited a higher abundance of leptin-receptor-expressing mesenchymal stromal cells (LepR-MSCs) compared with female mice. Sex-mismatched coculture and BM transplantation showed that the male BM niche provided superior support for in vitro colony formation and in vivo hematopoietic engraftment. The cotransplantation of male stromal cells significantly enhanced engraftment in female recipients. Single-cell RNA-seq revealed that the lower expression of the X-linked lysine H3K4 demethylase, Kdm5c, in male MSCs led to the increased expression of Cxcl12. In MSC-specific Kdm5c-KO mouse model, the reduction of KDM5C in female MSCs enhanced MSC quantity and function, ultimately improving engraftment to the male level. Kdm5c thus plays a role in driving sexual dimorphism in the BM niche and hematopoietic regeneration. Our study unveils a sex-dependent mechanism governing the BM niche regulation and its impact on hematopoietic engraftment. The finding offers potential implications for enhancing BM transplantation efficacy in clinical settings by harnessing the resource of male MSCs or targeting Kdm5c.
Stress Granules Underlie Acute Myeloid Leukemia Stem Cell Survival and Stress Adaptation
Preprint on BioRxiv : the Preprint Server for Biology on 17 January 2025 by Tajik, A., Tsao, E., et al.
ABSTRACT The link between cancer maintenance and an ability to sustain continued growth through stresses conferred by the cancer state itself is growing. However, there are significant gaps in our understanding of how this stress is managed, particularly at the level of cancer initiating cells. Here, we identify proteins comprising the dynamic, stress-adaptive ribonucleoprotein complexes known as stress granules (SG) to be enriched among the factors essential for leukemic stem cell (LSC)-driven leukemic propagation. Focusing on core SG nucleator G3BP1, we dissect the role of SGs in human acute myeloid leukemia (AML), their targetability, and the mechanisms they govern to uncover a novel propensity for AML, and in particular LSC-enriched fractions, to prime the expression of SG components, form SGs with greater fidelity and to be reliant on their establishment and continued integrity for LSC maintenance. We further unveil the transcript and protein interactome of G3BP1 in the AML context and show that consolidated control of innate immune signaling, and apoptosis repression is executed through regional binding specificity of G3BP1 to highly structured 3’UTRs and cooperation with the RNA helicase UPF1 to mediate transcript decay in SGs. Altogether our findings advance novel fundamental principles of stress adaptation exploited in AML and LSCs that may extend to other cancers and uncover SGs as a novel axis for therapy development.
-
Mus musculus (House mouse)
-
Cancer Research
-
Stem Cells and Developmental Biology
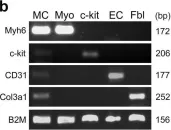
In NPJ Regen Med on 6 January 2018 by Czarna, A., Sanada, F., et al.
Fig.1.B

-
WB
-
Collected and cropped from NPJ Regen Med by CiteAb, provided under a CC-BY license
Image 1 of 1