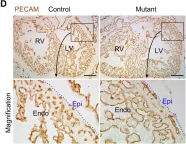
The cranial base synchondroses, comprised of opposite-facing bidirectional chondrocyte layers, drive anteroposterior cranial base growth. In humans, RUNX2 haploinsufficiency causes cleidocranial dysplasia associated with deficient midfacial growth. However, how RUNX2 regulates chondrocytes in the cranial base synchondroses remains unknown. To address this, we inactivated Runx2 in postnatal synchondrosis chondrocytes using a tamoxifen-inducible Fgfr3-creER (Fgfr3-Runx2cKO) mouse model. Fgfr3-Runx2cKO mice displayed skeletal dwarfism and reduced anteroposterior cranial base growth associated with premature synchondrosis ossification due to impaired chondrocyte proliferation, accelerated hypertrophy, apoptosis, and osteoclast-mediated cartilage resorption. Lineage tracing reveals that Runx2-deficient Fgfr3+ cells failed to differentiate into osteoblasts. Notably, Runx2-deficient chondrocytes showed an elevated level of FGFR3 and its downstream signaling components, pERK1/2 and SOX9, suggesting that RUNX2 downregulates FGFR3 in the synchondrosis. This study unveils a new role of Runx2 in cranial base chondrocytes, identifying a possible RUNX2-FGFR3-MAPK-SOX9 signaling axis that may control cranial base growth.
© 2025. The Author(s).
Product Citations: 980
RUNX2 is essential for maintaining synchondrosis chondrocytes and cranial base growth.
In Bone Research on 29 May 2025 by Hallett, S. A., Dixon, A., et al.
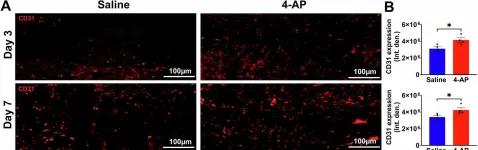
In International Journal of Medical Sciences on 19 May 2025 by Du, Z., Yu, F., et al.
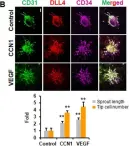
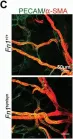
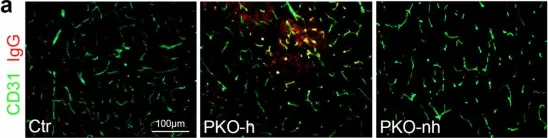
Introduction: Venous malformations (VMs) are congenital vascular malformations characterized by venous cavity enlargement and malformation. Although TIE2 mutation is a recognized genetic landscape in VMs, the regulatory role of TIE2 in vascular smooth muscle cell (VSMC) contraction remains unclear. Materials and Methods: We generated Tie2-R848Wfl/fl;Tie2Cre+ and Tie2-R848Wfl/fl;AplnER+ mice through specific expression of Tie2-R848W, a typical mutation in inherited VM, in endothelial cells (ECs). Histological and transcriptome sequencing analyses were performed on vascular abnormalities in the mutant mouse model. Postnatal vascular development in vivo was studied through morphometric analysis of the retinal vasculature. Under in vitro coculture conditions, the functional abnormality of VSMCs was studied using transwell analysis, proliferation analysis, a cell contraction assay and transcriptome sequencing analysis. Markers related to the VSMC phenotypic transition were analyzed via western blotting and quantitative RT‑PCR. Results: Tie2-R848Wfl/fl;Tie2Cre+ mice developed spontaneous pulmonary vascular malformations displaying internal hemorrhage and increased vasculature with α-SMA+ enveloped VSMCs. In Tie2-R848Wfl/fl;AplnER+ mice, Tie2-R848W mutation also induced postnatal retinal vascular malformations (higher vascular density and coverage of α-SMA+ VSMCs). According to phenotypes and molecular markers (Acta2, Cnn1, Sm22a and Opn), dysregulated phenotypic transition of VSMCs might be the pathogenic basis. Under in vitro coculture condition, the decreased contractile ability of synthetic VSMCs was significant in the mutant group, while downregulated ion transmembrane transport and TNFSF10 may play substantial roles in initiating this process. Conclusion: Endothelial TIE2 mutation might induce an abnormal EC-VSMC regulatory relationship strongly associated with phenotypic transition of VSMCs. Weakened contractility and abnormal proliferation induce chronic cavity expansion and thickening of the muscle layer, which may be potential mechanism basis of VMs.
© The author(s).
-
Cardiovascular biology
Dynamic cytoskeletal regulation of cell shape supports resilience of lymphatic endothelium.
In Nature on 1 May 2025 by Schoofs, H., Daubel, N., et al.
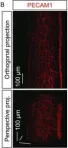
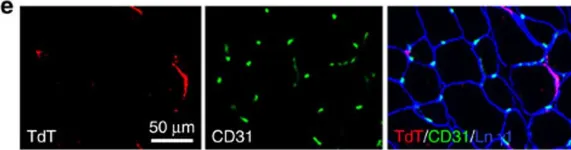
Lymphatic capillaries continuously take up interstitial fluid and adapt to resulting changes in vessel calibre1-3. The mechanisms by which the permeable monolayer of loosely connected lymphatic endothelial cells (LECs)4 maintains mechanical stability remain elusive. Here we identify dynamic cytoskeletal regulation of LEC shape, induced by isotropic stretch, as crucial for the integrity and function of dermal lymphatic capillaries. We found that the oak leaf-shaped LECs showed a spectrum of VE-cadherin-based junctional configurations at the lobular intercellular interface and a unique cytoskeletal organization, with microtubules at concave regions and F-actin at convex lobes. Multispectral and longitudinal intravital imaging of capillary LEC shape and actin revealed dynamic remodelling of cellular overlaps in vivo during homeostasis and in response to interstitial fluid volume increase. Akin to puzzle cells of the plant epidermis5,6, LEC shape was controlled by Rho GTPase CDC42-regulated cytoskeletal dynamics, enhancing monolayer stability. Moreover, cyclic isotropic stretch increased cellular overlaps and junction curvature in primary LECs. Our findings indicate that capillary LEC shape results from continuous remodelling of cellular overlaps that maintain vessel integrity while preserving permeable cell-cell contacts compatible with vessel expansion and fluid uptake. We propose a bellows-like fluid propulsion mechanism, in which fluid-induced lumen expansion and shrinkage of LEC overlaps are countered by actin-based lamellipodia-like overlap extension to aid vessel constriction.
© 2025. The Author(s).
-
Cell Biology
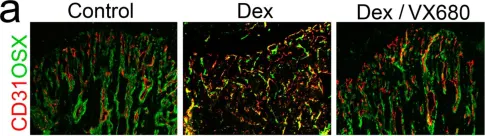
Desmoplastic tumor priming using clinical-stage corticosteroid liposomes.
In Cell Biomaterials on 22 April 2025 by Ojha, T., Schaefer, G. J. L., et al.
Inflammation is a hallmark of cancer. It contributes to a heterogeneous, hyperpermeable, and poorly perfused tumor vasculature, as well as to a dense and disorganized extracellular matrix, which together negatively affect drug delivery. Reasoning that glucocorticoids have pleiotropic effects, we use clinical-stage dexamethasone liposomes (LipoDex) to prime the tumor microenvironment for improved drug delivery and enhanced treatment efficacy. We show that LipoDex priming improves tumor vascular function and reduces extracellular matrix deposition. Single-cell sequencing corroborates LipoDex-mediated inhibition of pro-inflammatory, pro-angiogenic, and pro-fibrogenic gene expression in mononuclear cells, tumor-associated macrophages, and cancer-associated fibroblasts. Multimodal optical imaging illustrates that LipoDex pre-treatment increases the tumor accumulation and intratumoral distribution of subsequently administered polymeric and liposomal drug delivery systems. Using Doxil as a prototypic nanodrug, we finally show that LipoDex priming promotes antitumor treatment efficacy. Altogether, our findings demonstrate that desmoplastic tumors can be primed for improved drug targeting and therapy using clinical-stage glucocorticoid liposomes.
© 2025 The Author(s).
-
Cancer Research
β-III tubulin identifies anti-fibrotic state of pericytes in pulmonary fibrosis
Preprint on BioRxiv : the Preprint Server for Biology on 19 April 2025 by Sato, R., Imamura, K., et al.
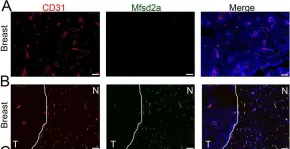
Pericytes have been implicated in pulmonary fibrosis, yet their activated cellular states and functional roles remain largely unclear. Here, we identified β-III tubulin (Tuj1) as a distinctive marker for fibrosis-associated lung pericytes. Most pericytes in fibrotic regions are Tuj1-positive and interact uniquely with multiple endothelial cells, localizing near collagen-producing fibroblasts and pro-fibrotic SPP1 + /arginase + macrophages. Tuj1 expression is predominantly induced in pericytes during the fibrotic phase, and Tuj1 gene ( Tubb3 ) knockout in mice exacerbates lung fibrosis, accompanied by an increase in the neighboring pro-fibrotic fibroblasts and macrophages, suggesting an anti-fibrotic role for Tuj1-expressing pericytes. Mechanistically, the anti-fibrotic chemokine CXCL10 is upregulated in Tuj1-expressing pericytes, whereas this upregulation is not observed in Tubb3 knockout. Moreover, CXCL10 inhibits the pro-fibrotic differentiation of macrophages induced by lung fibroblasts in culture, implying that CXCL10 may mediate the anti-fibrotic effects of Tuj1-expressing pericytes. These findings underscore the role of lung pericytes in negatively regulating fibrotic process and their potential as therapeutic targets for pulmonary fibrosis patients. Summary In response to fibrotic stimuli, lung pericytes upregulate β-III tubulin (Tuj1) expression, adopting an anti-fibrotic phenotype. This phenotype acts as a negative immune regulator of pro-fibrotic macrophage differentiation by releasing CXCL10, thereby counteracting the progression of pulmonary fibrosis.
-
Cardiovascular biology
-
Cell Biology
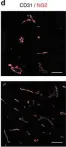
In Cell Death Discov on 4 October 2024 by V G, R., Ellur, G., et al.
Fig.4.A

-
IHC-IF
-
Collected and cropped from Cell Death Discov by CiteAb, provided under a CC-BY license
Image 1 of 21
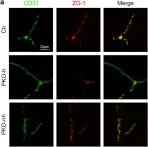
In Cells on 11 October 2023 by Qiao, X., Yang, Y., et al.
Fig.5.A

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 21
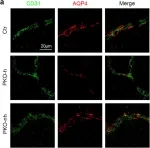
In Sci Rep on 12 June 2023 by Pepino, L., Malapert, P., et al.
Fig.4.B

-
ICC-IF
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 21
In Neurophotonics on 1 July 2022 by Freitas-Andrade, M., Comin, C. H., et al.
Fig.3.A

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Neurophotonics by CiteAb, provided under a CC-BY license
Image 1 of 21
In Neurophotonics on 1 July 2022 by Freitas-Andrade, M., Comin, C. H., et al.
Fig.3.B

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Neurophotonics by CiteAb, provided under a CC-BY license
Image 1 of 21
In Nat Commun on 19 May 2022 by Mece, O., Houbaert, D., et al.
Fig.7.H

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 21
In Cell Death Dis on 8 February 2022 by Kim, O., Hwangbo, C., et al.
Fig.8.G

-
IHC
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 21
In Cancers (Basel) on 27 May 2021 by Prabhakar, N., Merisaari, J., et al.
Fig.10.A

-
IHC-IF
-
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 21
In Sci Rep on 19 February 2020 by Ichikawa, K., Watanabe Miyano, S., et al.
Fig.4.E

-
IHC-IF
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 21
In Elife on 20 August 2019 by Park, M. H., Kim, A. K., et al.
Fig.2.B

-
ICC-IF
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 21
In Protein Cell on 1 February 2019 by Zhou, N., Liu, K., et al.
Fig.1.B

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Protein Cell by CiteAb, provided under a CC-BY license
Image 1 of 21
In Sci Rep on 29 May 2018 by Tiwary, S., Morales, J. E., et al.
Fig.2.A

-
IHC-IF
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 21
In Nat Commun on 18 July 2017 by Teichert, M., Milde, L., et al.
Fig.6.A

-
IF
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 21
In Nat Commun on 18 July 2017 by Teichert, M., Milde, L., et al.
Fig.6.D

-
IF
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 21
In Elife on 16 January 2017 by Benito-Jardón, M., Klapproth, S., et al.
Fig.1.C

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 21
In Sci Rep on 3 November 2016 by Gautam, J., Zhang, X., et al.
Fig.6.A

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 21
In Sci Rep on 3 November 2016 by Gautam, J., Zhang, X., et al.
Fig.7.A

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 21
In Sci Rep on 3 November 2016 by Gautam, J., Zhang, X., et al.
Fig.2.A

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 21
In Nat Commun on 3 May 2016 by Yao, Y., Norris, E. H., et al.
Fig.2.E

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 21
In PLoS One on 10 October 2014 by He, L., Tian, X., et al.
Fig.4.D

-
IHC
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 21
In PLoS One on 19 November 2008 by Caiado, F., Real, C., et al.
Fig.1.C

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 21