White adipocytes serve as primary energy reservoirs and their malfunction is linked to different metabolic disorders, yet the mechanisms underlying cellular specialization, a critical step during adipogenesis remain unknown. Here, we reveal the indispensable role of cutaneous T-cell lymphoma-associated antigen 5 (cTAGE5) in adipocyte differentiation and maturation. Conditional deletion of cTAGE5 in adipocyte precursor cells (APCs), rather than mature adipocytes, results in progressive loss of white adipose tissue and death of mice. Mechanistically, cTAGE5 deficiency in APCs disturbs pro-insulin receptor (IR) processing and impairs insulin signaling, accompanied by significant down-regulation of actin cytoskeleton related genes and defect in cytoskeleton remodeling, alongside enhanced expression of proteins associated with lipid catabolic process and lipolysis in adipocytes. Importantly, inhibitors targeting actin polymerization and lipolysis effectively restore adipocyte differentiation capacity in cTAGE5-deficient APCs. Collectively, our findings demonstrate that cTAGE5 plays pivotal roles in adipogenesis and adipose tissue development.
© 2025. The Author(s).
Product Citations: 178
cTAGE5 is essential for adipogenesis and adipose tissue development.
In Nature Communications on 1 July 2025 by Fan, J., Ma, T., et al.
-
FC/FACS
-
Mus musculus (House mouse)
In Cell Communication and Signaling : CCS on 13 March 2025 by Sivakumar, S., Lieber, S., et al.
High expression of basal cell adhesion molecule (BCAM) is a hallmark of ovarian cancer (OC) progression. BCAM facilitates transcoelomic dissemination by promoting mesothelial cell clearance at peritoneal attachment sites of tumor cell spheroids. We investigated how BCAM mediates this effect and potentially drives other pro-metastatic functions.
The impact of BCAM on the tumor cell secretome and the mesothelial cell phenotype was analyzed by affinity proteomics, bulk and single-cell RNA sequencing, life-cell and multiphoton microscopy, biochemical and functional in vitro assays as well as a murine tumor model. BCAM manipulation involved ectopic overexpression, inducible expression and treatment with soluble BCAM.
All forms of BCAM enhanced the secretion of cytokines that impact cell motility, mesenchymal differentiation and angiogenesis, including AREG, CXCL family members, FGF2, TGFB2, and VEGF. Notably, their levels in OC ascites were correlated with BCAM expression, and recombinant BCAM-induced cytokines triggered mesothelial-mesenchymal transition (MMT). Mesothelial cells undergoing MMT exhibited enhanced motility away from attaching tumor spheroids, leading to mesothelial clearance at spheroid attachment sites. BCAM-mediated MMT-associated transcriptional changes were also observed in subpopulations of omental mesothelial cells from OC patients, and were associated with poor survival. Consistent with the secretome data, BCAM induced endothelial tube formation in vitro and markedly promoted tumor angiogenesis in a mouse model.
We have identified previously unknown functions of the BCAM-induced secretome potentially impacting distinct stages of OC metastasis. While BCAM's impact on MMT may facilitate initiation of micrometastases, neo-angiogenesis is essential for tumor growth. Taken together with the observed clinical adverse association, our findings underscore the potential of BCAM as a therapeutic target.
© 2025. The Author(s).
-
Cancer Research
-
Endocrinology and Physiology
Impact of liver fibrosis on AAV-mediated gene transfer to mouse hepatocytes.
In Nature Communications on 10 March 2025 by Ferriero, R., Bruno, G., et al.
Liver fibrosis, characterized by scar tissue accumulation due to liver injury, poses significant barriers to liver-targeted gene therapy. Current clinical trials exclude patients with fibrosis, as intact liver architecture is considered essential for efficient and safe adeno-associated viral vector (AAV)-mediated gene delivery. Here, we show that liver fibrosis reduces the efficiency of hepatocyte transduction by AAV8 vectors across three mouse models with diverse fibrotic patterns. This inefficiency stems primarily from decreased vector uptake by the liver rather than loss of vector genomes due to hepatocyte turnover. Additionally, fibrosis alters blood vector clearance and redistributes AAV particles to extra-hepatic organs, such as spleen, lung, and kidney. At the cellular level, fibrosis decreases AAV genome content in hepatocytes while increasing it in non-parenchymal liver cells and splenic immune cells. Importantly, the capsid variant AAV-KP1 retains transduction efficiency in fibrotic livers, highlighting its potential for expanding gene therapy applications to fibrotic diseases.
© 2025. The Author(s).
In IScience on 17 January 2025 by Kiesworo, K., Agius, T., et al.
Aging is accompanied by a decline in neovascularization potential and increased susceptibility to ischemic injury. Here, we confirm the age-related impaired neovascularization following ischemic leg injury and impaired angiogenesis. The age-related deficits in angiogenesis arose primarily from diminished EC proliferation capacity, but not migration or VEGF sensitivity. Aged EC harvested from the mouse skeletal muscle displayed a pro-angiogenic gene expression phenotype, along with considerable changes in metabolic genes. Metabolomics analysis and 13C glucose tracing revealed impaired ATP production and blockade in glycolysis and TCA cycle in late passage HUVECs, which occurred at nicotinamide adenine dinucleotide (NAD⁺)-dependent steps, along with NAD+ depletion. Supplementation with nicotinamide mononucleotide (NMN), a precursor of NAD⁺, enhances late-passage EC proliferation and sprouting angiogenesis from aged mice aortas. Taken together, our study illustrates the importance of NAD+-dependent metabolism in the maintenance of EC proliferation capacity with age, and the therapeutic potential of NAD precursors.
© 2024 The Author(s).
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
PTPN1/2 inhibition promotes muscle stem cell differentiation in Duchenne muscular dystrophy.
In Life Science Alliance on 1 January 2025 by Liu, Y., Li, S., et al.
Duchenne muscular dystrophy (DMD) is a lethal disease caused by mutations in the DMD gene that encodes dystrophin. Dystrophin deficiency also impacts muscle stem cells (MuSCs), resulting in impaired asymmetric stem cell division and myogenic commitment. Using MuSCs from DMD patients and the DMD mouse model mdx, we found that PTPN1 phosphatase expression is up-regulated and STAT3 phosphorylation is concomitantly down-regulated in DMD MuSCs. To restore STAT3-mediated myogenic signaling, we examined the effect of K884, a novel PTPN1/2 inhibitor, on DMD MuSCs. Treatment with K884 enhanced STAT3 phosphorylation and promoted myogenic differentiation of DMD patient-derived MuSCs. In MuSCs from mdx mice, K884 treatment increased the number of asymmetric cell divisions, correlating with enhanced myogenic differentiation. Interestingly, the pro-myogenic effect of K884 is specific to human and murine DMD MuSCs and is absent from control MuSCs. Moreover, PTPN1/2 loss-of-function experiments indicate that the pro-myogenic impact of K884 is mediated mainly through PTPN1. We propose that PTPN1/2 inhibition may serve as a therapeutic strategy to restore the myogenic function of MuSCs in DMD.
© 2024 Liu et al.
-
Mus musculus (House mouse)
-
Stem Cells and Developmental Biology
In Sci Rep on 4 April 2022 by Jumabay, M., Zhang, L., et al.
Fig.2.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 3
In Sci Rep on 4 April 2022 by Jumabay, M., Zhang, L., et al.
Fig.6.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 3
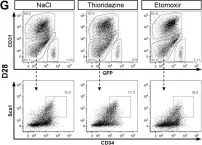
In J Cell Sci on 27 July 2018 by Pala, F., Di Girolamo, D., et al.
Fig.6.G

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from J Cell Sci by CiteAb, provided under a CC-BY license
Image 1 of 3