Lrba is a cytoplasmic protein involved in vesicular trafficking. Lrba-deficient (Lrba-/-) mice exhibit substantially higher levels of IgA in both serum and feces than wild-type (WT) mice. Transforming growth factor β1 (TGFβ1) and its receptors (TGFβR I and II) is essential for differentiating IgA+ B cells. Furthermore, increased IgA production suggests a potential connection between Lrba and the TGFβR signaling pathway in IgA production. However, the specific function of Lrba in B cell biology remains unknown.
Given the increased IgA levels in Lrba-/- mice, the goal in this work was to explore the lymph organs where the switch to IgA occurs, and if TGFβR function is affected.
Non-immunized Lrba-/- mice were compared with Lrba+/+ mice. IgA levels in the serum and feces, as well as during peripheral B cell development, were determined. IgA+ B cells and plasma cells were assessed in the small intestine and secondary lymphoid organs, such as the spleen, mesenteric lymph nodes, and Peyer's patches. The TGFβR signaling pathway was evaluated by determining the expression of TGFβR on B cells. Additionally, SMAD2 phosphorylation was measured under basal conditions and in response to recombinant TGFβ. Finally, confocal microscopy was performed to investigate a possible interaction between Lrba and TGFβR in B cells.
Lrba-/- mice exhibited significantly higher levels of circulating IgA, IgA+ B, and plasma cells than in peripheral lymphoid organs those in WT mice. TGFβR expression on the membrane of B cells was similar in both Lrba-/- and Lrba+/+ mice. However, intracellular TGFβR expression was reduced in Lrba-/- mice. SMAD2 phosphorylation showed increased levels under basal conditions; stimulation with recombinant TGFβ elicited a poorer response than in that in Lrba+/+ B cells. Finally, we found that Lrba colocalizes with TGFβR in B cells.
Lrba is essential in controlling TGFβR signaling, subsequently regulating SMAD2 phosphorylation on B cells. This mechanism may explain the increased differentiation of IgA+ B cells and production of IgA-producing plasma cells.
Copyright © 2024 Flores-Hermenegildo, Hernández-Cázares, Pérez-Pérez, Romero-Ramírez, Rodríguez-Alba, Licona-Limon, Kilimann, Santos-Argumedo and López-Herrera.
Product Citations: 48
Lrba participates in the differentiation of IgA+ B lymphocytes through TGFβR signaling.
In Frontiers in Immunology on 8 July 2024 by Flores-Hermenegildo, J. M., Hernández-Cázares, F. J., et al.
-
IHC-IF
-
Immunology and Microbiology
In Frontiers in Cellular and Infection Microbiology on 17 July 2023 by Hernández-Cázares, F., Maqueda-Alfaro, R. A., et al.
Patients with Human Hyper IgM syndromes (HIGM) developed pulmonary and gastrointestinal infections since infancy and most patients have mutations in the CD40 ligand (CD40L) gene. Most HIGM patients compared to healthy subjects have higher/similar IgM and lower IgG, and IgA serum concentrations but gut antibody concentrations are unknown. CD40L on activated T-cells interacts with CD40 on B-cells, essential for the formation of germinal centres (GCs) inside secondary lymphoid organs (SLOs), where high-affinity antibodies, long-lived antibody-secreting plasma cells, and memory B-cells, are produced. C57BL6-CD40 ligand deficient mice (C57BL6-cd40l -/-), are a model of HIGM, because serum immunoglobulin concentrations parallel levels observed in HIGM patients and have higher faecal IgA concentrations. In mice, TGFβ and other cytokines induce IgA production.
To compare and evaluate B-cell populations and IgA-producing plasma cells in peritoneal lavage, non-gut-associated SLOs, spleen/inguinal lymph nodes (ILN), and gut-associated SLOs, mesenteric lymph nodes (MLN)/Peyer´s patches (PP) of unimmunised C57BL6-cd40l -/- and C57BL6-wild-type (WT) mice.
Peritoneal lavages, spleens, ILN, MLN, and PP from 8-10 weeks old C57BL6-cd40l -/- and WT mice, were obtained. Organ cryosections were analysed by immunofluorescence and B-cell populations and IgA-positive plasma cell suspensions by flow cytometry.
In unimmunised WT mice, GCs were only observed in the gut-associated SLOs, but GCs were absent in all C57BL6-cd40l -/- SLOs. PP and MLN of C57BL6-cd40l -/- mice exhibited a significantly higher number of IgA-producing cells than WT mice. In the spleen and ILN of C57BL6-cd40l- /- mice IgA-producing cells significantly decreased, while IgM-positive plasma cells increased. C57BL6-cd40l -/- B-1 cells were more abundant in all analysed SLOs, whereas in WT mice most B-1 cells were contained within the peritoneal cavity. C57BL6-cd40l -/- B-cells in MLN expressed a higher TGFβ receptor-1 than WT mice. Mouse strains small intestine microvilli (MV), have a similar frequency of IgA-positive cells.
Together our results confirm the role of PP and MLN as gut inductive sites, whose characteristic features are to initiate an IgA preferential immune response production in these anatomical sites even in the absence of GCs. IgA antibodies play a pivotal role in neutralising, eliminating, and regulating potential pathogens and microorganisms in the gut.
Copyright © 2023 Hernandez-Cazares, Maqueda-Alfaro, Lopez-Saucedo, Martinez-Barnetche, Yam-Puc, Estrada-Parra, Flores-Romo and Estrada-Garcia.
-
Immunology and Microbiology
In IScience on 21 April 2023 by Marcial-Juárez, E., Pérez-Toledo, M., et al.
Germinal centers (GCs) are sites where plasma and memory B cells form to generate high-affinity, Ig class-switched antibodies. Specialized stromal cells called follicular dendritic cells (FDCs) are essential for GC formation. During systemic Salmonella Typhimurium (STm) infection GCs are absent, whereas extensive extrafollicular and switched antibody responses are maintained. The mechanisms that underpin the absence of GC formation are incompletely understood. Here, we demonstrate that STm induces a reversible disruption of niches within the splenic microenvironment, including the T and B cell compartments and the marginal zone. Alongside these effects after infection, mature FDC networks are strikingly absent, whereas immature FDC precursors, including marginal sinus pre-FDCs (MadCAM-1+) and perivascular pre-FDCs (PDGFRβ+) are enriched. As normal FDC networks re-establish, extensive GCs become detectable throughout the spleen. Therefore, the reorganization of FDC networks and the loss of GC responses are key, parallel features of systemic STm infections.
© 2023 The Authors.
-
Immunology and Microbiology
Preprint on Research Square on 27 June 2022 by Gazzinelli, R., de Castro, J. T., et al.
We have systematically shown that the Amastigote Surface Protein-2 (ASP-2) and Transialidase (TS) antigens either in the form of recombinant protein or encoded in the plasmids and human adenovirus 5 (hAd5) confer strong protection against different lineages of Trypanosoma cruzi parasites. Herein we generated a chimeric protein containing the most immunogenic regions for T and B cells from ASP-2 and TS (DTT-1) and evaluated its efficacy in comparison with our standard protocol of heterologous prime-boost using plasmids and hAd5. Our results show that mice immunized with DTT-1 protein together with Poly-ICLC (Hiltonol) become highly resistant to challenge with the Y strain of T. cruzi, showing a large decrease in tissue parasitism, parasitemia and no lethality. This protection lasted for at least 3 months after the last boost of immunization and an equivalent level of protection induced by the DNA/hAd5 protocol. DTT-1 induced high levels of T. cruzi-specific antibodies and IFNγ-producing T cells and protection was mediated by B lymphocytes, CD8 T cells and IFNγ. Finally, we evaluated the toxicity, immunogenicity and efficacy of the DTT-1 and DNA/hAd5 formulations in dogs. Mild collateral effects were detected at the site of vaccine inoculation. While the chimeric protein associated to Poly-ICLC induced high levels of antibodies and CD4+ T cell responses, the DNA/hAd5 induced no antibodies, but a strong CD8+ T cell response. Immunization with either vaccine formulations protected dogs against challenge with the Berenice strain of T. cruzi. Despite to the similar efficacy, we conclude that moving ahead with DTT-1 together with Hiltonol is advantageous over the hAd5 vaccine due to the cost-benefit for development and large-scale production.
-
Immunology and Microbiology
-
Veterinary Research
In Frontiers in Immunology on 10 September 2021 by Szumilas, N., Corneth, O. B. J., et al.
Siglec-H is a DAP12-associated receptor on plasmacytoid dendritic cells (pDCs) and microglia. Siglec-H inhibits TLR9-induced IFN-α production by pDCs. Previously, it was found that Siglec-H-deficient mice develop a lupus-like severe autoimmune disease after persistent murine cytomegalovirus (mCMV) infection. This was due to enhanced type I interferon responses, including IFN-α. Here we examined, whether other virus infections can also induce autoimmunity in Siglec-H-deficient mice. To this end we infected Siglec-H-deficient mice with influenza virus or with Lymphocytic Choriomeningitis virus (LCMV) clone 13. With both types of viruses we did not observe induction of autoimmune disease in Siglec-H-deficient mice. This can be explained by the fact that both types of viruses are ssRNA viruses that engage TLR7, rather than TLR9. Also, Influenza causes an acute infection that is rapidly cleared and the chronicity of LCMV clone 13 may not be sufficient and may rather suppress pDC functions. Siglec-H inhibited exclusively TLR-9 driven type I interferon responses, but did not affect type II or type III interferon production by pDCs. Siglec-H-deficient pDCs showed impaired Hck expression, which is a Src-family kinase expressed in myeloid cells, and downmodulation of the chemokine receptor CCR9, that has important functions for pDCs. Accordingly, Siglec-H-deficient pDCs showed impaired migration towards the CCR9 ligand CCL25. Furthermore, autoimmune-related genes such as Klk1 and DNase1l3 are downregulated in Siglec-H-deficient pDCs as well. From these findings we conclude that Siglec-H controls TLR-9-dependent, but not TLR-7 dependent inflammatory responses after virus infections and regulates chemokine responsiveness of pDCs.
Copyright © 2021 Szumilas, Corneth, Lehmann, Schmitt, Cunz, Cullen, Chu, Marosan, Mócsai, Benes, Zehn, Dudziak, Hendriks and Nitschke.
-
Immunology and Microbiology
In Mol Cancer on 9 November 2005 by Han, S. S., Shaffer, A. L., et al.
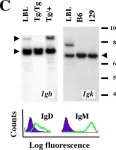
Fig.1.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Mol Cancer by CiteAb, provided under a CC-BY license
Image 1 of 1