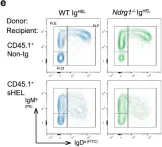
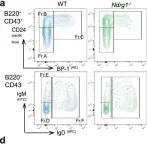
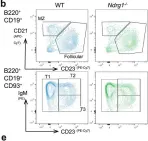
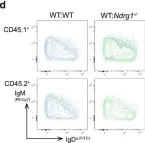
Peripheral tolerance prevents the initiation of damaging immune responses by autoreactive lymphocytes. While tolerogenic mechanisms are tightly regulated by antigen-dependent and independent signals, downstream pathways are incompletely understood. N-myc downstream-regulated gene 1 (NDRG1), an anti-cancer therapeutic target, has previously been implicated as a CD4+ T cell clonal anergy factor. By RNA-sequencing, we identified Ndrg1 as the third most upregulated gene in anergic, compared to naïve follicular, B cells. Ndrg1 is upregulated by B cell receptor activation (signal one) and suppressed by co-stimulation (signal two), suggesting that NDRG1 may be important in B cell tolerance. However, though Ndrg1-/- mice have a neurological defect mimicking NDRG1-associated Charcot-Marie-Tooth (CMT4d) disease, primary and secondary immune responses were normal. We find that B cell tolerance is maintained, and NDRG1 does not play a role in downstream responses during re-stimulation of in vivo antigen-experienced CD4+ T cells, demonstrating that NDGR1 is functionally redundant for lymphocyte anergy.
© 2022. The Author(s).
Product Citations: 24
NDRG1 is induced by antigen-receptor signaling but dispensable for B and T cell self-tolerance.
In Communications Biology on 10 November 2022 by Hodgson, R., Xu, X., et al.
-
ELISA
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Cell Reports on 14 June 2022 by Worth, A. N., Palmer, V. L., et al.
B1 B cells reactive to phosphatidyl choline (PtC) exhibit restricted immunoglobulin heavy chain (HC) and light chain (LC) combinations, exemplified by VH12/Vκ4/5H. Two checkpoints are thought to focus PtC+ B cell maturation in VH12-transgenic mice (VH12 mice): V-J rearrangements encoding a "permissive" LC capable of VH12 HC pairing are selected first, followed by positive selection based on PtC binding, often requiring LC receptor editing to salvage PtC- B cells and acquire PtC reactivity. However, evidence obtained from breeding VH12 mice to editing-defective dnRAG1 mice and analyzing LC sequences from PtC+ and PtC- B cell subsets instead suggests that receptor editing functions after initial positive selection to remove PtC+ B cells in VH12 mice. This offers a mechanism to constrain natural, polyreactive B cells to limit their frequency. Sequencing also reveals occasional in-frame hybrid LC genes, reminiscent of type 2 gene replacement, that, testing suggests, arise via a recombination-activating gene (RAG)-independent mechanism.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
In Frontiers in Immunology on 10 April 2020 by Grødeland, G., Baranowska-Hustad, M., et al.
Novel and more broadly protective vaccines against influenza are needed to efficiently meet antigenic drift and shift. Relevant to this end, the stem domain of hemagglutinin (HA) is highly conserved, and antibodies specific for epitopes located to the stem have been demonstrated to be able to confer broad protection against various influenza subtypes. However, a remaining challenge is to induce antibodies against the poorly immunogenic stem by vaccination strategies that can be scaled up for prophylactic vaccination of the general population. Here, we have developed DNA vaccines where the conserved stem domain of HA from influenza A/PR/8/34 (H1N1) and A/Shanghai/2/2013 (H7N9) was targeted toward MHC class II molecules on antigen-presenting cells (APC) for increased immunogenicity. Each of these vaccines induced antibodies that cross-reacted with other subtypes in the corresponding phylogenetic influenza groups. Importantly, when mixing the MHCII-targeted stem domains from H1N1 and H7N9 influenza viruses into one vaccine bolus, we observed broad protection against candidate stains from both phylogenetic groups 1 and 2.
Copyright © 2020 Grødeland, Baranowska-Hustad, Abadejos, Blane, Teijaro, Nemazee and Bogen.
-
ELISA
-
Mus musculus (House mouse)
-
Genetics
-
Immunology and Microbiology
In NPJ Vaccines on 22 December 2017 by Stephen, J., Scales, H. E., et al.
There are over 6 billion vaccine doses administered each year, most containing aluminium-based adjuvants, yet we still do not have a complete understanding of their mechanisms of action. Recent evidence has identified host DNA and downstream sensing as playing a significant role in aluminium adjuvant (aluminium hydroxide) activity. However, the cellular source of this DNA, how it is sensed by the immune system and the consequences of this for vaccination remains unclear. Here we show that the very early injection site reaction is characterised by inflammatory chemokine production and neutrophil recruitment. Intravital imaging demonstrates that the Alum injection site is a focus of neutrophil swarms and extracellular DNA strands. These strands were confirmed as neutrophil extracellular traps due to their sensitivity to DNAse and absence in mice deficient in peptidylarginine deiminase 4. Further studies in PAD4-/- mice confirmed a significant role for neutrophil extracellular trap formation in the adjuvant activity of Alum. By revealing neutrophils recruited to the site of Alum injection as a source of the DNA that is detected by the immune system this study provides the missing link between Alum injection and the activation of DNA sensors that enhance adjuvant activity, elucidating a key mechanism of action for this important vaccine component.
-
FC/FACS
-
Mus musculus (House mouse)
Identification of autoreactive B cells with labeled nucleosomes.
In Scientific Reports on 4 April 2017 by Gies, V., Wagner, A., et al.
The pathogenesis of autoimmune diseases has not been completely elucidated yet, and only a few specific treatments have been developed so far. In autoimmune diseases mediated by pathogenic autoantibodies, such as systemic lupus erythematosus, the specific detection and analysis of autoreactive B cells is crucial for a better understanding of the physiopathology. Biological characterization of these cells may help to define new therapeutic targets. Very few techniques allowing the precise detection of autoreactive B cells have been described so far. Herein we propose a new flow cytometry technique for specific detection of anti-nucleosome B cells, which secrete autoantibodies in systemic lupus erythematosus, using labeled nucleosomes. We produced different fluorochrome-labeled nucleosomes, characterized them, and finally tested them in flow cytometry. Nucleosomes labeled via the cysteines present in H3 histone specifically bind to autoreactive B cells in the anti-DNA transgenic B6.56R mice model. The present work validates the use of fluorochrome-labeled nucleosomes via cysteines to identify anti-nucleosome B cells and offers new opportunities for the description of autoreactive B cell phenotype.
-
Immunology and Microbiology
In Commun Biol on 10 November 2022 by Hodgson, R., Xu, X., et al.
Fig.4.E
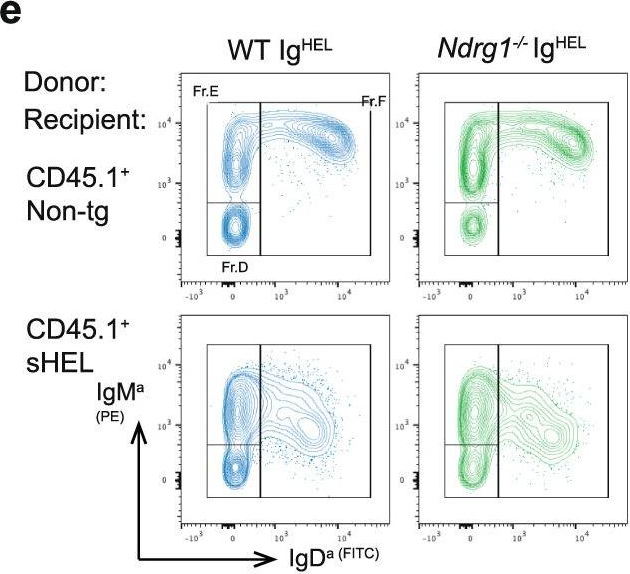
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Commun Biol by CiteAb, provided under a CC-BY license
Image 1 of 4
In Commun Biol on 10 November 2022 by Hodgson, R., Xu, X., et al.
Fig.3.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Commun Biol by CiteAb, provided under a CC-BY license
Image 1 of 4
In Commun Biol on 10 November 2022 by Hodgson, R., Xu, X., et al.
Fig.3.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Commun Biol by CiteAb, provided under a CC-BY license
Image 1 of 4
In Commun Biol on 10 November 2022 by Hodgson, R., Xu, X., et al.
Fig.5.D
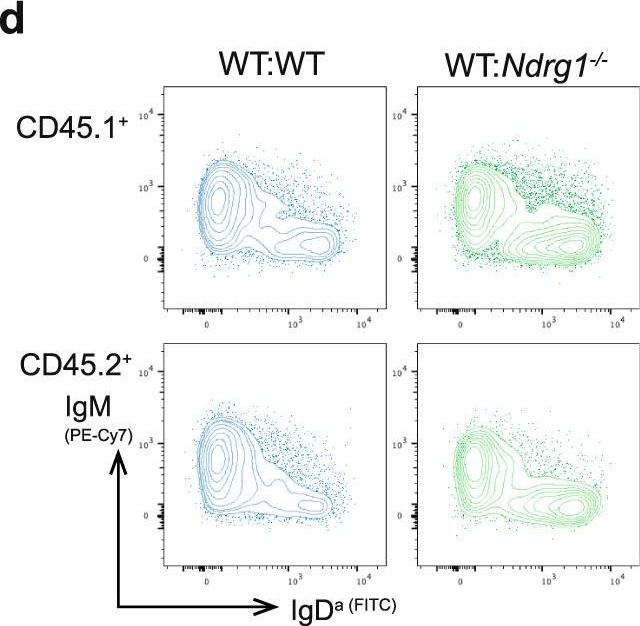
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Commun Biol by CiteAb, provided under a CC-BY license
Image 1 of 4