Antigen binding by B cell receptors (BCR) on cognate B cells elicits a response that eventually leads to production of antibodies. However, it is unclear what the distribution of BCRs is on the naïve B cell and how antigen binding triggers the first step in BCR signaling. Using DNA-PAINT super-resolution microscopy, we find that most BCRs are present as monomers, dimers, or loosely associated clusters on resting B cells, with a nearest-neighbor inter-Fab distance of 20-30 nm. We leverage a Holliday junction nanoscaffold to engineer monodisperse model antigens with precision-controlled affinity and valency, and find that the antigen exerts agonistic effects on the BCR as a function of increasing affinity and avidity. Monovalent macromolecular antigens can activate the BCR at high concentrations, whereas micromolecular antigens cannot, demonstrating that antigen binding does not directly drive activation. Based on this, we propose a BCR activation model determined by the antigen footprint.
© 2023. The Author(s).
Product Citations: 16
Antigen footprint governs activation of the B cell receptor.
In Nature Communications on 22 February 2023 by Ferapontov, A., Omer, M., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
NDRG1 is induced by antigen-receptor signaling but dispensable for B and T cell self-tolerance.
In Communications Biology on 10 November 2022 by Hodgson, R., Xu, X., et al.
Peripheral tolerance prevents the initiation of damaging immune responses by autoreactive lymphocytes. While tolerogenic mechanisms are tightly regulated by antigen-dependent and independent signals, downstream pathways are incompletely understood. N-myc downstream-regulated gene 1 (NDRG1), an anti-cancer therapeutic target, has previously been implicated as a CD4+ T cell clonal anergy factor. By RNA-sequencing, we identified Ndrg1 as the third most upregulated gene in anergic, compared to naïve follicular, B cells. Ndrg1 is upregulated by B cell receptor activation (signal one) and suppressed by co-stimulation (signal two), suggesting that NDRG1 may be important in B cell tolerance. However, though Ndrg1-/- mice have a neurological defect mimicking NDRG1-associated Charcot-Marie-Tooth (CMT4d) disease, primary and secondary immune responses were normal. We find that B cell tolerance is maintained, and NDRG1 does not play a role in downstream responses during re-stimulation of in vivo antigen-experienced CD4+ T cells, demonstrating that NDGR1 is functionally redundant for lymphocyte anergy.
© 2022. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Cell Reports on 3 May 2022 by Jing, Z., McCarron, M. J., et al.
T follicular helper (TFH) cells promote expansion of germinal center (GC) B cells and plasma cell differentiation. Whether cognate peptide-MHCII (pMHCII) density instructs selection and cell fate decisions in a quantitative manner remains unclear. Using αDEC205-OVA to differentially deliver OVA peptides to GC B cells on the basis of DEC205 allelic copy number, we find DEC205+/+ B cells take up 2-fold more antigen than DEC205+/- cells, leading to proportional TFH cell help and B cell expansion. To validate these results, we establish a caged OVA peptide, which is readily detected by OVA-specific TFH cells after photo-uncaging. In situ uncaging of peptides leads to multiple serial B-T contacts and cell activation. Differential CD40 signaling, is both necessary and sufficient to mediate 2-fold differences in B cell expansion. While plasmablast numbers are increased, pMHCII density does not directly control the output or quality of plasma cells. Thus, we distinguish the roles TFH cells play in expansion versus differentiation.Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
In Diabetes on 1 July 2016 by Leeth, C. M., Racine, J., et al.
While the autoimmune destruction of pancreatic ß-cells underlying type 1 diabetes (1D) development is ultimately mediated by T-cells in NOD mice and also likely humans, B-lymphocytes play an additional key pathogenic role. It appears expression of plasma membrane bound immunoglobulin (Ig) molecules that efficiently capture ß-cell antigens allows autoreactive B-lymphocytes bypassing normal tolerance induction processes to be the subset of antigen presenting cells most efficiently activating diabetogenic T-cells. NOD mice transgenically expressing Ig molecules recognizing antigens that are (insulin) or not (hen egg lysozyme; HEL) expressed by ß-cells have proven useful in dissecting the developmental basis of diabetogenic B-lymphocytes. However, these transgenic Ig specificities were originally selected for their ability to recognize insulin or HEL as foreign, rather than autoantigens. Thus, we generated and characterized NOD mice transgenically expressing an Ig molecule representative of a large proportion of naturally occurring islet-infiltrating B-lymphocytes in NOD mice recognizing the neuronal antigen peripherin. Transgenic peripherin autoreactive B-lymphocytes infiltrate NOD pancreatic islets, acquire an activated proliferative phenotype, and potently support accelerated T1D development. These results support the concept of neuronal autoimmunity as a pathogenic feature of T1D, and targeting such responses could ultimately provide an effective disease intervention approach.
© 2016 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
-
Immunology and Microbiology
In The Journal of Immunology on 15 September 2014 by Steinel, N. C., Fisher, M. R., et al.
Coordination of V rearrangements between loci on homologous chromosomes is critical for Ig and TCR allelic exclusion. The Ataxia Telangietasia mutated (ATM) protein kinase promotes DNA repair and activates checkpoints to suppress aberrant Ig and TCR rearrangements. In response to RAG cleavage of Igκ loci, ATM inhibits RAG expression and suppresses further Vκ-to-Jκ rearrangements to enforce Igκ allelic exclusion. Because V recombination between alleles is more strictly regulated for TCRβ and IgH loci, we evaluated the ability of ATM to restrict biallelic expression and V-to-DJ recombination of TCRβ and IgH genes. We detected greater frequencies of lymphocytes with biallelic expression or aberrant V-to-DJ rearrangement of TCRβ or IgH loci in mice lacking ATM. A preassembled DJβ complex that decreases the number of TCRβ rearrangements needed for a productive TCRβ gene further increased frequencies of ATM-deficient cells with biallelic TCRβ expression. IgH and TCRβ proteins drive proliferation of prolymphocytes through cyclin D3 (Ccnd3), which also inhibits VH transcription. We show that inactivation of Ccnd3 leads to increased frequencies of lymphocytes with biallelic expression of IgH or TCRβ genes. We also show that Ccnd3 inactivation cooperates with ATM deficiency to increase the frequencies of cells with biallelic TCRβ or IgH expression while decreasing the frequency of ATM-deficient lymphocytes with aberrant V-to-DJ recombination. Our data demonstrate that core components of the DNA damage response and cell cycle machinery cooperate to help enforce IgH and TCRβ allelic exclusion and indicate that control of V-to-DJ rearrangements between alleles is important to maintain genomic stability.Copyright © 2014 by The American Association of Immunologists, Inc.
-
Immunology and Microbiology
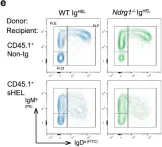
In Commun Biol on 10 November 2022 by Hodgson, R., Xu, X., et al.
Fig.4.E
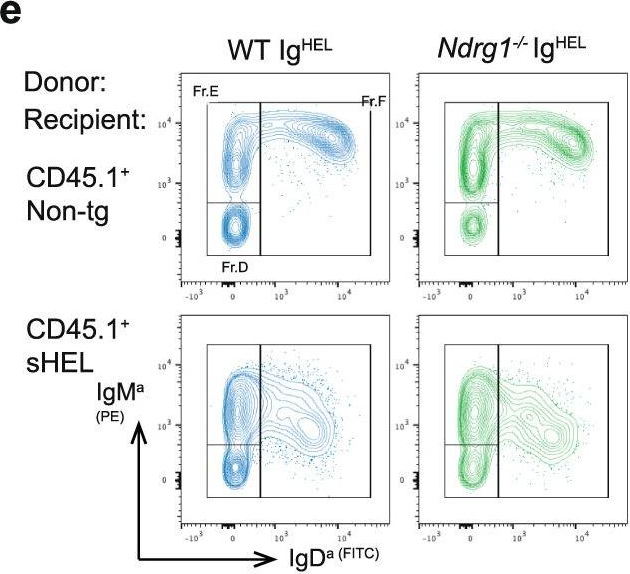
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Communications Biology by CiteAb, provided under a CC-BY license
Image 1 of 4
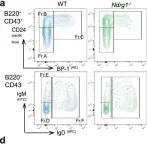
In Commun Biol on 10 November 2022 by Hodgson, R., Xu, X., et al.
Fig.3.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Communications Biology by CiteAb, provided under a CC-BY license
Image 1 of 4
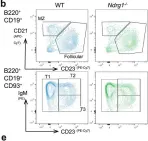
In Commun Biol on 10 November 2022 by Hodgson, R., Xu, X., et al.
Fig.3.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Communications Biology by CiteAb, provided under a CC-BY license
Image 1 of 4
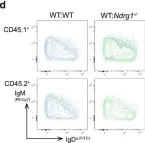
In Commun Biol on 10 November 2022 by Hodgson, R., Xu, X., et al.
Fig.5.D
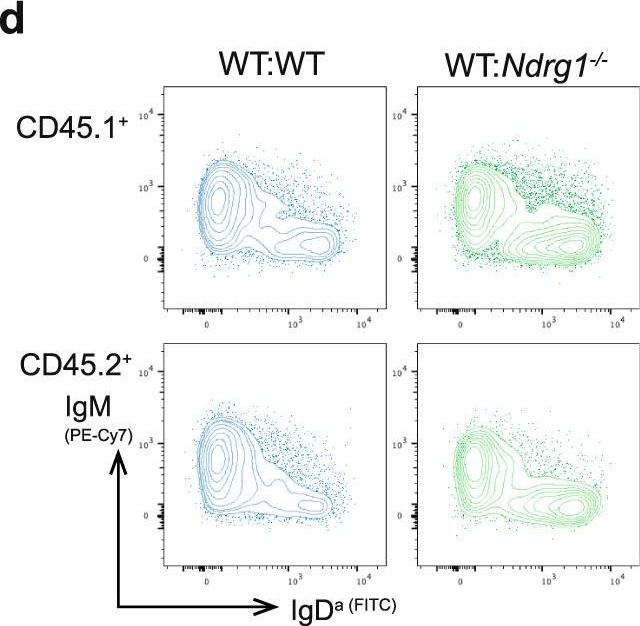
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Communications Biology by CiteAb, provided under a CC-BY license
Image 1 of 4