Human genetic studies implicate interleukin-27 (IL-27) in the pathogenesis of type 1 diabetes (T1D), but the underlying mechanisms remain largely unexplored. To further define the role of IL-27 in T1D, we generated non-obese diabetic (NOD) mice deficient in IL-27 or IL-27Rα. In contrast to wild-type NOD mice, both NOD.Il27-/- and NOD.Il27ra-/- strains are completely resistant to T1D. IL-27 from myeloid cells and IL-27 signaling in T cells are critical for T1D development. IL-27 directly alters the balance of regulatory T cells (Tregs) and T helper 1 (Th1) cells in pancreatic islets, which in turn modulates the diabetogenic activity of CD8 T cells. IL-27 also directly enhances the effector function of CD8 T cells within pancreatic islets. In addition to T1D, IL-27 signaling in T cells is also required for lacrimal and salivary gland inflammation in NOD mice. Our study reveals that IL-27 contributes to autoimmunity in NOD mice through multiple mechanisms and provides substantial evidence to support its pathogenic role in human T1D.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Product Citations: 21
Interleukin-27 Is Essential for Type 1 Diabetes Development and Sjögren Syndrome-like Inflammation.
In Cell Reports on 3 December 2019 by Ciecko, A. E., Foda, B., et al.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
BPTF inhibits NK cell activity and the abundance of natural cytotoxicity receptor co-ligands.
In Oncotarget on 8 September 2017 by Mayes, K., Elsayed, Z., et al.
Using syngeneic BALB/c mouse breast cancer models, we show that the chromatin remodeling subunit bromodomain PHD finger transcription factor (BPTF) suppresses natural killer (NK) cell antitumor activity in the tumor microenvironment (TME). In culture, BPTF suppresses direct natural cytotoxicity receptor (NCR) mediated NK cell cytolytic activity to mouse and human cancer cell lines, demonstrating conserved functions. Blocking mouse NCR1 in vivo rescues BPTF KD tumor weights, demonstrating its importance for the control of tumor growth. We discovered that BPTF occupies heparanase (Hpse) regulatory elements, activating its expression. Increased heparanase activity results in reduced cell surface abundance of the NCR co-ligands: heparan sulfate proteoglycans (HSPGs). Using gain and loss of function approaches we show that elevated heparanase levels suppress NK cell cytolytic activity to tumor cells in culture. These results suggest that BPTF activates heparanase expression, which in turn reduces cell surface HSPGs and NCR co-ligands, inhibiting NK cell activity. Furthermore, gene expression data from human breast cancer tumors shows that elevated BPTF expression correlates with reduced antitumor immune cell signatures, supporting conserved roles for BPTF in suppressing antitumor immunity. Conditional BPTF depletion in established mouse breast tumors enhances antitumor immunity, suggesting that inhibiting BPTF could provide a novel immunotherapy.
-
FC/FACS
-
Mus musculus (House mouse)
In Frontiers in Immunology on 15 July 2015 by Vogel, A. J. & Brown, D. M.
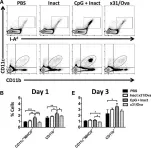
Despite extensive research, influenza A virus (IAV) remains a major cause of morbidity, mortality, and healthcare expenditure. Emerging pandemics from highly pathogenic IAV strains, such as H5N1 and pandemic H1N1, highlight the need for universal, cross-protective vaccines. Current vaccine formulations generate strain-specific neutralizing antibodies primarily against the outer coat proteins, hemagglutinin and neuraminidase. In contrast to these highly mutable proteins, internal proteins of IAV are more conserved and are a favorable target for developing vaccines that induce strong T cell responses in addition to humoral immunity. Here, we found that intranasal administration with a single dose of CpG and inactivated x31 (H3N2) reduced viral titers and partially protected mice from a heterosubtypic challenge with a lethal dose of PR8 (H1N1). Early after immunization, vaccinated mice showed increased innate immune activation with high levels of MHCII and CD86 expression on dendritic cells in both draining lymph nodes and lungs. Three days after immunization, CD4 and CD8 cells in the lung upregulated CD69, suggesting that activated lymphocytes are present at the site of vaccine administration. The ensuing effector Th1 responses were capable of producing multiple cytokines and were present at least 30 days after immunization. Furthermore, functional memory responses were observed, as antigen-specific IFN-γ(+) and GrB(+) cells were detected early after lethal infection. Together, this work provides evidence for using pattern recognition receptor agonists as a mucosal vaccine platform for inducing robust T cell responses capable of protecting against heterologous IAV challenges.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Preemptive Tolerogenic Delivery of Donor Antigens for Permanent Allogeneic Islet Graft Protection.
In Cell Transplantation on 25 April 2014 by Wang, S., Zhang, X., et al.
We have previously developed a robust regimen for tolerance induction in murine models of islet cell transplantation using pre- and posttransplant infusions of donor splenocytes (SPs) treated with a chemical cross-linker ethylcarbodiimide (ECDI). However, the requirement for large numbers of fresh donor SPs for ECDI coupling impairs its clinical feasibility, and additionally, the compatibility of this tolerance regimen with commonly used immunosuppressive drugs is largely unknown. In the current study, we demonstrate that equivalent tolerance efficacy for islet cell transplantation can be successfully achieved not only with a significantly lower dose of ECDI-SPs than originally established but also with culture-expanded donor B-cells or with soluble donor antigens in the form of donor cell lysate, which is ECDI coupled to recipient SPs. We further demonstrate that tolerance induced by donor ECDI-SPs is dependent on a favorable apoptotic-to-necrotic cell ratio post-ECDI coupling and is not affected by a transient course of conventional immunosuppressive drugs including tacrolimus and mycophenolate mofetil. While splenic antigen-presenting cells of the recipient play an important role in mediating the tolerogenic effects of donor ECDI-SPs, splenectomized recipients can be readily tolerized and appear to employ liver Kupffer cells for uptaking and processing of the ECDI-SPs. We conclude that infusion of donor ECDI-SPs is a versatile tolerance strategy that has a high potential for adaptation to clinically feasible regimens for tolerance trials for human islet cell transplantation.
In Oncoimmunology on 1 December 2013 by Llopiz, D., Huarte, E., et al.
Peptide vaccines derived from CD8+ T-cell epitopes have shown variable efficacy in cancer patients. Thus, some peptide vaccines are capable of activating CD8+ T-cell responses, even in the absence of CD4+ T-cell epitopes or dendritic cell (DC)-activating adjuvants. However, the mechanisms underlying the clinical activity of these potent peptides are poorly understood. Using CT26 and ovalbumin-expressing B16 murine allograft tumor models, we found that the antitumor effect of helper cell-independent CD8 T-cell peptide vaccines is inhibited by the blockade of CD40 ligand (CD40L) in vivo. Furthermore, in vitro stimulation with antigenic peptides of cells derived from immunized mice induced the expression of CD40L on the surface of CD8+ T cells and fostered DC maturation, an effect that was partially inhibited by CD40L-blocking antibodies. Interestingly, CD40L blockade also inhibited CD8+ T-cell responses, even in the presence of fully mature DCs, suggesting a role for CD40L not only in promoting DC maturation but also in mediating CD8+ T-cell co-stimulation. Importantly, these potent peptides share features with bona fide CD4 epitopes, since they foster responses against less immunogenic CD8+ T-cell epitopes in a CD40L-dependent manner. The analysis of peptides used for the vaccination of cancer patients in clinical trials showed that these peptides also induce the expression of CD40L on the surface of CD8+ T cells. Taken together, these results suggest that CD40L expression induced by potent CD8+ T-cell epitopes can activate antitumor CD8+ T-cell responses, potentially amplifying the immunological responses to less immunogenic CD8+ T-cell epitopes and bypassing the requirement for CD4+ helper T cells in vaccination protocols.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Front Immunol on 15 July 2015 by Vogel, A. J. & Brown, D. M.
Fig.3.A,B,E

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1