Obesity is a major driver of type 2 diabetes (T2D) and related metabolic disorders, characterized by chronic inflammation and adipocyte dysfunction. However, the molecular triggers initiating these processes remain poorly understood. We identify FAM20C, a serine/threonine kinase, as an early obesity-induced mediator of adipocyte dysfunction. Fam20c expression is substantially upregulated in adipocytes in response to obesity, correlating with a proinflammatory transcriptional signature. Forced expression of Fam20c in adipocytes promotes robust upregulation of proinflammatory cytokines and induces insulin resistance that is dependent on its kinase activity. Conversely, deletion of adipocyte Fam20c after established obesity and hyperglycemia improves glucose tolerance, augments insulin sensitivity, and reduces visceral adiposity, without altering body weight. Phosphoproteomic studies reveal that FAM20C regulates phosphorylation of intracellular and secreted proteins, modulating pathways critical to inflammation, metabolism, and extracellular matrix remodeling. We identify FAM20C-dependent substrates, such as CNPY4, whose phosphorylation contributes to proinflammatory adipocyte signaling. Of translational relevance, we show that in humans visceral adipose FAM20C expression positively correlates with insulin resistance. Our findings establish FAM20C as an early regulator of obesity-induced adipocyte dysfunction and systemic metabolic impairment. Our studies provide proof of concept that inhibition of FAM20C may serve as a potential therapy for T2D by restoring adipocyte health.
Product Citations: 33
In The Journal of Clinical Investigation on 28 October 2025 by Gilani, A., Stein, B. D., et al.
-
FC/FACS
-
Endocrinology and Physiology
-
Immunology and Microbiology
In Cell Reports Medicine on 21 October 2025 by Su, N., Lian, W., et al.
Tumor immune microenvironment greatly influences triple-negative breast cancer (TNBC) progression. Identifying targets to convert "cold" tumors into "hot" tumors holds promise for improving treatment outcomes. Here, we show that high expression of NEDD4, an HECT-type E3 ubiquitin ligase, correlates with poor prognosis and reduced CD8+ T cell infiltration in TNBC patients. NEDD4 depletion in TNBC cells significantly inhibits tumor growth through enhancing CD8+ T cell-mediated cytotoxicity in immunocompetent hosts. Mechanistically, NEDD4 depletion stabilizes β-TrCP, leading to YAP ubiquitination and degradation. Downregulated YAP reprograms the immunosuppressive tumor extracellular matrix (ECM) to increase CD8+ T cell infiltration. Furthermore, a small-molecule inhibitor of NEDD4, XMU-MP-10, exhibits significant in vivo efficacy in inhibiting TNBC tumor growth by enhancing CD8+ T cell infiltration in mouse models. Collectively, our findings suggest that the genetic depletion or pharmacological inhibition of NEDD4 enhances antitumor immune responses via the β-TrCP/YAP/ECM cascades, offering a promising therapeutic strategy for TNBC treatment.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
-
FC/FACS
-
Cancer Research
-
Immunology and Microbiology
In ACS Nano on 21 October 2025 by Shanmugam, M., Chiang, C. S., et al.
The discovery of immune checkpoint inhibitor (ICI) therapies was granted the Nobel Prize in 2018. However, ICI immunotherapies were later found working poorly for the large majority (70-80%) of cancer patients. It is an urgent need to develop a strategy to conquer this grand challenge and reverse otherwise ineffective immunotherapies to become effective. Herein, we propose a "Blind T cells" model to well rationalize the course leading to the ineffectiveness of immunotherapies. We demonstrate an effective strategy to conquer the ineffectiveness of immunotherapies via producing a large amount of newly generated tumor-recognizing cytolytic CD8+ T cells before the administration of immunotherapy reagents. We apply a NIR-IV photodynamic therapy, mediated by LaB6-PEG-folate nanoparticles using 2240 nm NIR light excitation, to generate reactive oxygen species, kill cancer cells, in situ produce whole cancer vaccines for priming of CD8+ T cells, and induce immunogenic responses in the presence of immunomodulator anti-OX40. A multifunctional anti-OX40 agonist could co-stimulate naive T cells to proliferate with tumor-recognizing properties, as well as suppress the activities of immunosuppressive Treg, and M2-phenotype macrophages, resulting in the complete disappearance of the primary melanoma tumor (that exposes to NIR light irradiation) as well as the effective suppression of remote/metastatic tumors' growths in the lung (that did not receive photo-irradiation).
-
FC/FACS
-
Cancer Research
-
Immunology and Microbiology
In Journal of Neuroinflammation on 26 August 2025 by Hui, R., Xu, J., et al.
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
In Frontiers in Immunology on 28 January 2025 by Xu, S., Chen, J., et al.
Epidemiological investigations have revealed a significant association between alcohol consumption and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). Nevertheless, the potential mechanisms are still inadequately revealed. This research aimed to investigate the impact of alcohol on CP/CPPS using an animal model and to elucidate the underlying mechanisms.
We first established the widely used animal model for CP/CPPS, experimental autoimmune prostatitis (EAP). During the induction of EAP, mice were fed with alcohol or control diet. The HE staining, ELISA, and behavioral experiments were employed to assess the severity of inflammation in EAP mice and EAP-alcohol mice. Patients with a history of chronic alcohol consumption were also included to evaluate the effects of chronic alcohol consumption on CP/CPPS. Subsequently, proteomic analysis, flow cytometry, immunofluorescence, Western blotting, and immunohistochemistry were utilized to investigate the underlying mechanism involved both in vivo and in vitro.
HE staining, ELISA, and behavioral experiments showed that alcohol exacerbated the severity of EAP in mice and patients. Proteomic and KEGG pathway analyses showed that abnormal Th1 differentiation and PI3K/AKT/mTOR pathway were significantly enriched. Subsequent mechanistic research showed that alcohol significantly activated PI3K/AKT/mTOR pathway and increased the Th1 cell differentiation both in vivo and in vitro. In contrast, PI3K inhibitor LY294002 and shRNA-PI3K plasmid inhibited PI3K/AKT/mTOR pathway activation, reduced Th1 cell differentiation, and alleviated EAP inflammation severity, respectively.
Our study is the first to demonstrate that alcohol intake promotes Th1 cell differentiation and exacerbates EAP by activating the PI3K/AKT/mTOR pathway. Additionally, the role of LY294002 in inhibiting PI3K/AKT/mTOR pathway to relieve EAP suggests that it can serve as a promising therapeutic target for CP/CPPS.
Copyright © 2025 Xu, Chen, Yue, Zhang, Zhao, Hu, Zhang, Guan, Zhang, Zhang and Liang.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
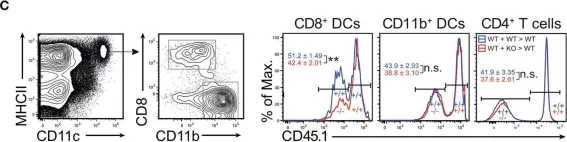
In Front Immunol on 28 February 2019 by Zwick, M., Ulas, T., et al.
Fig.4.C
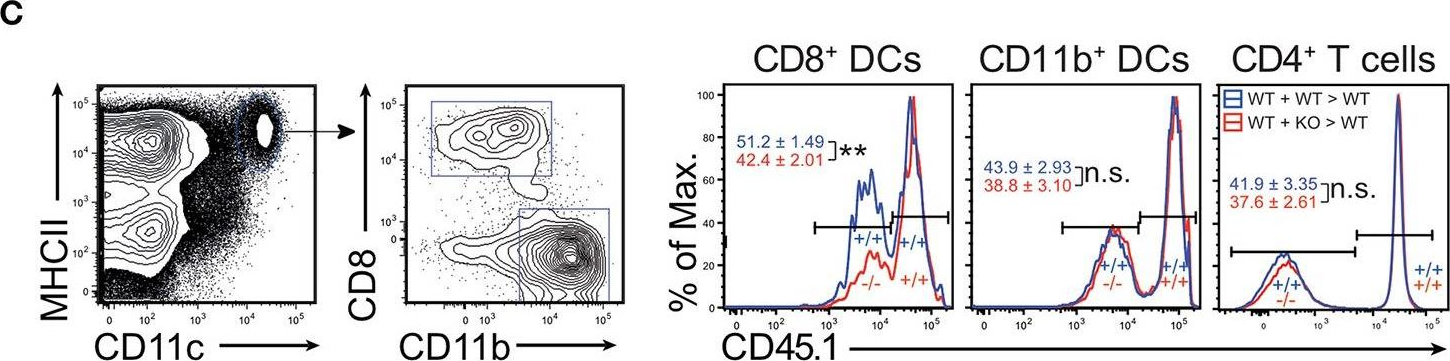
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Frontiers in Immunology by CiteAb, provided under a CC-BY license
Image 1 of 1