The recent emergence of a causal link between Epstein-Barr virus (EBV) and multiple sclerosis has generated considerable interest in the development of an effective vaccine against EBV. Here we describe a vaccine formulation based on a lymph node targeting Amphiphile vaccine adjuvant, Amphiphile-CpG, admixed with EBV gp350 glycoprotein and an engineered EBV polyepitope protein that includes 20 CD8+ T cell epitopes from EBV latent and lytic antigens. Potent gp350-specific IgG responses are induced in mice with titers >100,000 in Amphiphile-CpG vaccinated mice. Immunization including Amphiphile-CpG also induces high frequencies of polyfunctional gp350-specific CD4+ T cells and EBV-specific CD8+ T cells that are 2-fold greater than soluble CpG and are maintained for >7 months post immunization. This combination of broad humoral and cellular immunity against multiple viral determinants is likely to provide better protection against primary infection and control of latently infected B cells leading to protection against the development of EBV-associated diseases.
© 2023. The Author(s).
Product Citations: 87
In Nature Communications on 8 August 2023 by Dasari, V., McNeil, L. K., et al.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Ceramide Nanoliposomes as Potential Therapeutic Reagents for Asthma.
In Cells on 11 February 2023 by Sakae, H., Ogiso, Y., et al.
Ceramides are an emerging class of anti-inflammatory lipids, and nanoscale ceramide-delivery systems are potential therapeutic strategies for inflammatory diseases. This study investigated the therapeutic effects of ceramide nanoliposomes (CNL) on type 2 inflammation-based asthma, induced by repeated ovalbumin (OVA) challenges. Asthmatic mice intratracheally treated with ceramide-free liposomes (Ghost) displayed typical airway remodeling including mucosal accumulation and subepithelial fibrosis, whereas, in CNL-treated mice, the degree of airway remodeling was significantly decreased. Compared to the Ghost group, CNL treatment unexpectedly failed to significantly influence formation of type 2 cytokines, including IL-5 and IL-13, known to facilitate pathogenic production of airway mucus predominantly comprising MUC5AC mucin. Interestingly, CNL treatment suppressed OVA-evoked hyperplasia of MUC5AC-generating goblet cells in the airways. This suggests that CNL suppressed goblet cell hyperplasia and airway mucosal accumulation independently of type 2 cytokine formation. Mechanistically, CNL treatment suppressed cell growth and EGF-induced activation of Akt, but not ERK1/2, in a human lung epithelial cell culture system recapitulating airway goblet cell hyperplasia. Taken together, CNL is suggested to have therapeutic effects on airway remodeling in allergic asthma by targeting goblet cell hyperplasia. These findings raise the potential of ceramide-based therapies for airway diseases, such as asthma.
-
Mus musculus (House mouse)
-
Cell Biology
Preprint on Research Square on 13 July 2022 by Dasari, V., McNeil, L., et al.
Recent emergence of a causal like between Epstein-Barr virus (EBV) and multiple sclerosis has generated considerable interest in the development of an effective vaccine against EBV. Here we describe a novel vaccine formulation based on a lymph node targeting Amphiphile (AMP) vaccine adjuvant, AMP-CpG, admixed with EBV gp350 glycoprotein and a novel EBV-polyepitope protein (EBVpoly) that includes 20 CD8 + T-cell epitopes from EBV latent and lytic antigens. Potent gp350-specific IgG responses were induced in mice with titers > 100,000 in AMP-CpG vaccinated mice. Immunization including AMP-CpG also induced high frequencies of polyfunctional gp350-specific CD4 + T-cells and EBVpoly-specific CD8 + T-cells that were 2-fold greater than soluble CpG and were maintained for > 7 months post immunization. This combination of broad humoral and cellular immunity against multiple viral determinants is likely to provide better protection against primary infection and control of latently infected B cells leading to protection against the development of EBV-associated diseases. Teaser A lymph node targeted AMP-CpG subunit EBV vaccine induces potent and durable immunity in HLA expressing mice.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Heliyon on 1 July 2022 by Kasahara, K., Sasaki, N., et al.
Compelling evidence suggests a crucial role for Foxp3+ regulatory T cells (Tregs) in the control of atherosclerosis. Although suppression of pro-inflammatory CD4+ T cell immune responses is supposed to be important for athero-protective action of Foxp3+ Tregs, few studies have provided direct evidence for this protective mechanism. We investigated the impact of Foxp3+ Treg depletion on CD4+ T cell immune responses and the development of atherosclerosis under hypercholesterolemia. We employed DEREG (depletion of regulatory T cells) mice on an atherosclerosis-prone low-density lipoprotein receptor-deficient (Ldlr -/-) background, which carry a diphtheria toxin (DT) receptor under the control of the foxp3 gene locus. In these mice, DT injection led to efficient depletion of Foxp3+ Tregs in spleen, lymph nodes and aorta. Depletion of Foxp3+ Tregs augmented CD4+ effector T cell immune responses and aggravated atherosclerosis without affecting plasma lipid profile. Notably, the proportion of pro-inflammatory IFN-γ-producing T cells were increased in spleen and aorta following Foxp3+ Treg depletion, implying that Foxp3+ Tregs efficiently regulate systemic and aortic T cell-mediated inflammatory responses under hypercholesterolemia. Unexpectedly, Foxp3+ Treg depletion resulted in an increase in anti-inflammatory IL-10-producing T cells, which was not sufficient to suppress the augmented proinflammatory T cell immune responses caused by reduced numbers of Foxp3+ Tregs. Our data indicate that Foxp3+ Tregs suppress pro-inflammatory CD4+ T cell immune responses to control atherosclerosis under hypercholesterolemia.
© 2022 The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Lung tumor MHCII immunity depends on in situ antigen presentation by fibroblasts.
In The Journal of Experimental Medicine on 7 February 2022 by Kerdidani, D., Aerakis, E., et al.
A key unknown of the functional space in tumor immunity is whether CD4 T cells depend on intratumoral MHCII cancer antigen recognition. MHCII-expressing, antigen-presenting cancer-associated fibroblasts (apCAFs) have been found in breast and pancreatic tumors and are considered to be immunosuppressive. This analysis shows that antigen-presenting fibroblasts are frequent in human lung non-small cell carcinomas, where they seem to actively promote rather than suppress MHCII immunity. Lung apCAFs directly activated the TCRs of effector CD4 T cells and at the same time produced C1q, which acted on T cell C1qbp to rescue them from apoptosis. Fibroblast-specific MHCII or C1q deletion impaired CD4 T cell immunity and accelerated tumor growth, while inducing C1qbp in adoptively transferred CD4 T cells expanded their numbers and reduced tumors. Collectively, we have characterized in the lungs a subset of antigen-presenting fibroblasts with tumor-suppressive properties and propose that cancer immunotherapies might be strongly dependent on in situ MHCII antigen presentation.
© 2022 Kerdidani et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
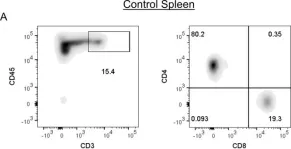
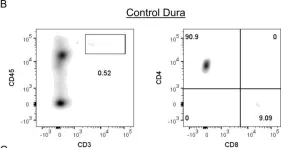
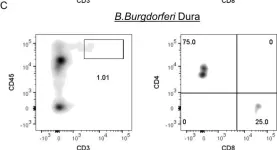
In PLoS One on 4 May 2018 by Divan, A., Casselli, T., et al.
Fig.4.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
In PLoS One on 4 May 2018 by Divan, A., Casselli, T., et al.
Fig.4.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
In PLoS One on 4 May 2018 by Divan, A., Casselli, T., et al.
Fig.4.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
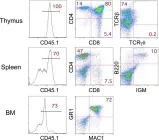
In Stem Cell Reports on 10 November 2015 by Ikawa, T., Masuda, K., et al.
Fig.2.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Stem Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 4