The myelination is a critical process during brain development. This study aimed to explore the impact of volatile anesthetic sevoflurane on developing myelination and the role of microglial activation in this process. Neonatal C57BL/6J mice were exposed to sevoflurane at their postnatal 6-8 days. Neurobehavioral tests were used to assess fine motor and cognitive functions. Myelination of hippocampus (HC) and corpus callosum (CC), as well as microglial activation, were determined by western blotting and immunostaining. Lipid droplets were assessed by Oil-Red-O and Bodipy staining. Further, primary microglia were co-cultured with oligodendrocyte precursor cell (OPC) to determine the role of microglia in the proliferation and differentiation of OPC. And microglial inhibitor minocycline and CSF1R inhibitor PLX5622 were administered to assess the effects of microglial activation on developing myelination. The results showed that repeated sevoflurane exposure impaired both fine motor and cognitive functions and induced abnormal expressions of myelin-related proteins myelin basic protein (MBP) and platelet-derived growth factor α receptor (PDGFR-α). And accumulations of lipid droplets were found in the microglia of HC and CC after sevoflurane exposure. Further, the spatiotemporal response to repeated sevoflurane exposure in glial cells exhibited an aberrant myelination process and microglial polarization. The conditioned medium from sevoflurane-treated microglia inhibited the OPC proliferation and differentiation, while minocycline or PLX5622 alleviated sevoflurane-induced neuroinflammation and hypomyelination. Therefore, repeated sevoflurane exposure negatively affected OPC differentiation and myelination trajectory through hyperactivating microglia in developing brain, leading to motor and cognitive impairments, while microglial inhibition/depletion could protect against sevoflurane-induced damage on developing myelination.
© 2025 Wiley Periodicals LLC.
Product Citations: 185
In GLIA on 1 June 2025 by Che, J., Wu, Y., et al.
-
Neuroscience
In CNS Neuroscience Therapeutics on 1 December 2024 by Bui, V. T., Wu, K. W., et al.
Hericium erinaceus mycelium and its constituents, erinacines A and S, have shown neuroprotective effects in APP/PS1 transgenic mice; however, the precise mechanisms by which they modulate microglial phenotypes remain unclear. Our study is the first to explore the effect of erinacines on microglia morphology and the underlying mechanisms using a novel primary mixed glia cell model and advanced bioinformatic tools. Furthermore, we emphasize the clinical relevance by evaluating erinacines in a metabolically stressed APP/PS1 mouse model, which more accurately reflects the complexities of human Alzheimer's disease (AD), where metabolic syndrome is a common comorbidity.
Rat primary mixed glial cultures were used to simulate the spectrum of microglial phenotypes, particularly the transition from immature to mature states. Microarray sequencing, along with Connectivity Map, ConsensusPathDB, and Gene Set Enrichment Analysis, identified pathways influenced by erinacines. The therapeutic efficacy was further evaluated in metabolically stressed APP/PS1 mice.
Erinacines significantly promoted the development of a ramified, neuroprotective microglial phenotype. Bioinformatics revealed potential modulation of microglia via histone deacetylase inhibition, actin filament dynamics, and synaptic structure modification-pathways not previously linked to erinacines in AD. Importantly, erinacines significantly lower fasting blood glucose and insulin levels while reducing amyloid-beta plaque burden, suppressing hyperactivated glial responses, and enhancing neurogenesis in the metabolically stressed APP/PS1 mice.
Our findings demonstrate the dual action of erinacines in modulating microglia morphology and phenotype while providing neuroprotection in a model that closely mimic the complexities of human Alzheimer's disease. Additionally, this study provides the foundation for understanding the potential mechanisms of action of erinacines, highlighting their promise as a novel treatment approach for Alzheimer's, particularly in cases complicated by metabolic dysfunction.
© 2024 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
-
Biochemistry and Molecular biology
-
Cell Biology
-
Neuroscience
-
Pathology
In CNS Neuroscience Therapeutics on 1 March 2024 by Che, J., Wang, H., et al.
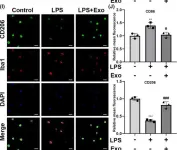
We investigated whether human umbilical cord mesenchymal stem cell (hUC-MSC)-derived exosomes bear therapeutic potential against lipopolysaccharide (LPS)-induced neuroinflammation.
Exosomes were isolated from hUC-MSC supernatant by ultra-high-speed centrifugation and characterized by transmission electron microscopy and western blotting. Inflammatory responses were induced by LPS in BV-2 cells, primary microglial cultures, and C57BL/6J mice. H2 O2 was also used to induce inflammation and oxidative stress in BV-2 cells. The effects of hUC-MSC-derived exosomes on inflammatory cytokine expression, oxidative stress, and microglia polarization were studied by immunofluorescence and western blotting.
Treatment with hUC-MSC-derived exosomes significantly decreased the LPS- or H2 O2 -induced oxidative stress and expression of pro-inflammatory cytokines (IL-6 and TNF-α) in vitro, while promoting an anti-inflammatory (classical M2) phenotype in an LPS-treated mouse model. Mechanistically, the exosomes increased the NRF2 levels and inhibited the LPS-induced NF-κB p65 phosphorylation and NLRP3 inflammasome activation. In contrast, the reactive oxygen species scavenger NAC and NF-κB inhibitor BAY 11-7082 also inhibited the LPS-induced NLRP3 inflammasome activation and switched to the classical M2 phenotype. Treatment with the NRF2 inhibitor ML385 abolished the anti-inflammatory and anti-oxidative effects of the exosomes.
hUC-MSC-derived exosomes ameliorated LPS/H2 O2 -induced neuroinflammation and oxidative stress by inhibiting the microglial NRF2/NF-κB/NLRP3 signaling pathway.
© 2023 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
-
ICC-IF
-
Mus musculus (House mouse)
-
Stem Cells and Developmental Biology
A subset of Kupffer cells regulates metabolism through the expression of CD36.
In Immunity on 14 September 2021 by Blériot, C., Barreby, E., et al.
Tissue macrophages are immune cells whose phenotypes and functions are dictated by origin and niches. However, tissues are complex environments, and macrophage heterogeneity within the same organ has been overlooked so far. Here, we used high-dimensional approaches to characterize macrophage populations in the murine liver. We identified two distinct populations among embryonically derived Kupffer cells (KCs) sharing a core signature while differentially expressing numerous genes and proteins: a major CD206loESAM- population (KC1) and a minor CD206hiESAM+ population (KC2). KC2 expressed genes involved in metabolic processes, including fatty acid metabolism both in steady-state and in diet-induced obesity and hepatic steatosis. Functional characterization by depletion of KC2 or targeted silencing of the fatty acid transporter Cd36 highlighted a crucial contribution of KC2 in the liver oxidative stress associated with obesity. In summary, our study reveals that KCs are more heterogeneous than anticipated, notably describing a subpopulation wired with metabolic functions.
Copyright © 2021 Elsevier Inc. All rights reserved.
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
In Biomedicines on 3 April 2021 by Garello, F., Boido, M., et al.
Labeling of macrophages with perfluorocarbon (PFC)-based compounds allows the visualization of inflammatory processes by 19F-magnetic resonance imaging (19F-MRI), due to the absence of endogenous background. Even if PFC-labeling of monocytes/macrophages has been largely investigated and used, information is lacking about the impact of these agents over the polarization towards one of their cell subsets and on the best way to image them. In the present work, a PFC-based nanoemulsion was developed to monitor the course of inflammation in a model of spinal cord injury (SCI), a pathology in which the understanding of immunological events is of utmost importance to select the optimal therapeutic strategies. The effects of PFC over macrophage polarization were studied in vitro, on cultured macrophages, and in vivo, in a mouse SCI model, by testing and comparing various cell tracking protocols, including single and multiple administrations, the use of MRI or Point Resolved Spectroscopy (PRESS), and application of pre-saturation of Kupffer cells. The blood half-life of nanoemulsion was also investigated by 19F Magnetic Resonance Spectroscopy (MRS). In vitro and in vivo results indicate the occurrence of a switch towards the M2 (anti-inflammatory) phenotype, suggesting a possible theranostic function of these nanoparticles. The comparative work presented here allows the reader to select the most appropriate protocol according to the research objectives (quantitative data acquisition, visual monitoring of macrophage recruitment, theranostic purpose, rapid MRI acquisition, etc.). Finally, the method developed here to determine the blood half-life of the PFC nanoemulsion can be extended to other fluorinated compounds.
-
Immunology and Microbiology
-
Neuroscience
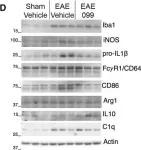
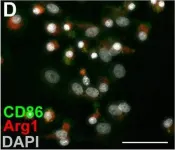
In CNS Neurosci Ther on 1 March 2024 by Che, J., Wang, H., et al.
Fig.3.I

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from CNS Neurosci Ther by CiteAb, provided under a CC-BY license
Image 1 of 8
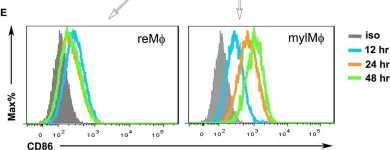
In Sci Rep on 11 June 2020 by Nakahara, T., Kido-Nakahara, M., et al.
Fig.5.B

-
FC/FACS
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 8
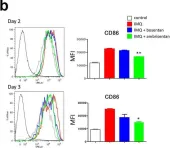
In Front Immunol on 4 April 2019 by Ban, Y., Dong, W., et al.
Fig.1.C

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 8
In Front Immunol on 4 April 2019 by Ban, Y., Dong, W., et al.
Fig.1.E

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 8
In Eneuro on 11 January 2019 by Bellizzi, M. J., Hammond, J. W., et al.
Fig.2.D

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Eneuro by CiteAb, provided under a CC-BY license
Image 1 of 8
In Elife on 16 May 2017 by Simkin, J., Gawriluk, T. R., et al.
Fig.5.D

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 8
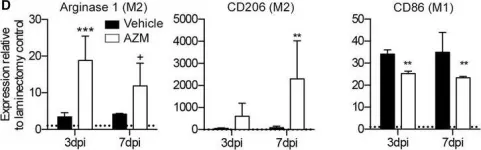
In J Neuroinflammation on 24 November 2015 by Zhang, B., Bailey, W. M., et al.
Fig.1.D

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from J Neuroinflammation by CiteAb, provided under a CC-BY license
Image 1 of 8
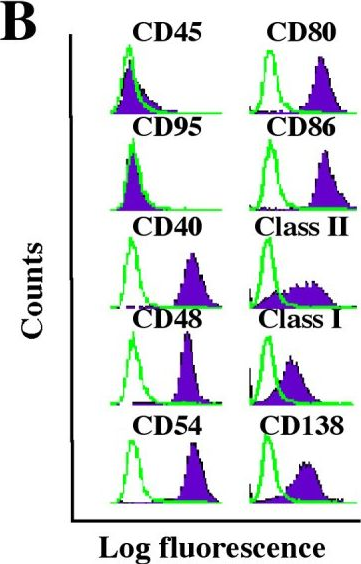
In Mol Cancer on 9 November 2005 by Han, S. S., Shaffer, A. L., et al.
Fig.1.B
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Mol Cancer by CiteAb, provided under a CC-BY license
Image 1 of 8