Macrophage pyroptosis has been identified as a critical pathological mechanism in inflammation-related atherosclerosis (AS). In this work, we have demonstrated that Zn2+ features the strongest anti-inflammatory performance by screening 10 representative metal ions, and the MTC1 agonists can trigger lysosomal Zn2+ release and inhibit pyroptosis in macrophages. Based on these findings, we further engineered a mucolipin TRP channel 1 (MTC1)-related therapeutic nanoplatform for endogenously triggering lysosomal zinc release to curb inflammation and block macrophage pyroptosis. This nanoplatform consists of mesoporous silica nanoparticles to deliver MTC1 agonists and carbon nanodots, which could synergistically exert antiatherosclerotic effect by scavenging toxic reactive oxygen species, inhibiting macrophage pyroptosis, modulating macrophage transition, and rebuilding atherosclerotic immune microenvironment. These findings demonstrate that macrophage pyroptosis can be efficiently blocked via leveraging self-lysosomal zinc pool, which provides the paradigm of lysosomal zinc modulation-involved nanotherapeutics for managing other inflammatory diseases.
Product Citations: 195
In Science Advances on 27 June 2025 by Hu, R., Qin, J., et al.
-
Cell Biology
-
Immunology and Microbiology
In Bone Research on 11 June 2025 by Rong, K., Wang, D., et al.
Itaconate, a macrophage-specific anti-inflammatory metabolite, has recently emerged as a critical regulator in rheumatoid arthritis pathogenesis. We found that itaconate is a TNF-α responsive metabolite significantly elevated in the serum and synovial fluid of rheumatoid arthritis patients and we demonstrated that itaconate is primarily produced by inflammatory macrophages rather than osteoclasts or osteoblasts. In TNF-transgenic and Irg1-/- hybrid mice, a more severe bone destruction phenotype was observed. Administration of itaconate prevents excessive activation of osteoclasts by inhibiting Tet2 enzyme activity. Furthermore, exogenous administration of itaconate or its derivative, 4-octyl-itaconate, inhibits arthritis progression and mitigates bone destruction, offering a potential therapeutic strategy for rheumatoid arthritis. This study elucidates that TNF-α drives macrophage-derived itaconate production to epigenetically suppress osteoclast hyperactivation through Tet2 inhibition, establishing itaconate and its derivative OI as novel therapeutic agents against rheumatoid arthritis -associated bone destruction.
© 2025. The Author(s).
-
FC/FACS
-
Genetics
-
Immunology and Microbiology
In ACS Nano on 8 April 2025 by Mo, X., shen, A., et al.
Macrophage plays critical roles in immune-related diseases, acting as a crucial therapeutic target for immunotherapy. Rational design and development of effective therapeutics for macrophage reprogramming are still challenging. Here, we rationally engineered polysaccharide nanoadjuvants to reprogram macrophage functions for enhanced immunotherapy in multiple diseases through a macrophage phenotype-specific nanoprobe (MPSNPr)-assisted high-throughput phenotypic screen. This MPSNPr exhibited high macrophage M1 phenotype specificity because of the formation of H-aggregates on the outer surface and the binding to glucose transporter 1 receptors by the polysaccharide nanocarrier. Based on this MPSNPr, a high-throughput platform was constructed and employed to screen a variety of pharmaceuticals for macrophage reprogramming, being able to identify both pro-inflammatory and anti-inflammatory drug candidates. Polysaccharide nanoadjuvants, Dex-BA and Dex-SAL, were rationally engineered with two potent candidates to amplify macrophage reprogramming efficacy both in vitro and in vivo. Dex-BA significantly inhibited tumor growth by inducing macrophage M1 polarization, dendritic cell maturation, and cytotoxic T cell activation in a mice melanoma model. Dex-SAL alleviated rheumatoid arthritis symptoms with reduced inflammation by reprogramming activated macrophages toward anti-inflammatory phenotype. Our work provides a robust strategy for the rational design and development of effective therapeutics for enhanced macrophage-mediated immunotherapy in diverse diseases.
-
Cancer Research
-
Immunology and Microbiology
Lymphatic platelet thrombosis limits bone repair by precluding lymphatic transporting DAMPs.
In Nature Communications on 18 January 2025 by Zheng, Y., Cong, L., et al.
In the musculoskeletal system, lymphatic vessels (LVs), which are interdigitated with blood vessels, travel and form an extensive transport network. Blood vessels in bone regulate osteogenesis and hematopoiesis, however, whether LVs in bone affect fracture healing is unclear. Here, we investigate the lymphatic draining function at the tibial fracture sites using near-infrared indocyanine green lymphatic imaging (NIR-ICG) and discover that lymphatic drainage insufficiency (LDI) starts on day one and persists for up to two weeks following the fracture in male mice. Sufficient lymphatic drainage facilitates fracture healing in male mice. Furthermore, we identify that lymphatic platelet thrombosis (LPT) blocks the draining lymphoid sinus and LVs, causes LDI, and inhibits fracture healing in male mice, which can be rescued by a blood thinner. Moreover, unblocked lymphatic drainage decreases neutrophils and increases M2-type macrophages of the hematoma niche to support osteoblast (OB) survival and bone marrow-derived mesenchymal stem cell (BMSC) proliferation via transporting damage-associated molecular patterns (DAMPs) in male rats. Lymphatic platelet thrombolysis also benefits senile fracture healing in female mice. These findings demonstrate that LPT limits bone regeneration by impeding lymphatic transporting DAMPs. Together, these findings represent a way forward in the treatment of bone repair.
© 2025. The Author(s).
-
Mus musculus (House mouse)
In The ISME Journal on 2 January 2025 by Wang, Y., Wang, X., et al.
Dysbiosis of gut microbiota plays a crucial role in acute radiation-induced intestinal injury. However, studies on the influence of gut microbiota on acute radiation-induced intestinal injury are inconsistent. In this study, we established an acute radiation-induced intestinal injury mouse model and performed fecal microbiota transplantation to explore the role of the gut microbiota in acute radiation-induced intestinal injury. We observed a significant increase in Akkermansia muciniphila following irradiation, whereas fecal microbiota transplantation effectively reduced A. muciniphila levels. Contrary to expectations, A. muciniphila supplementation increased acute radiation-induced intestinal injury and mortality. Mechanistically, postradiation A. muciniphila upregulates mucin metabolism genes and consumes mucin, thinning the mucosal barrier and promoting the adhesion and translocation of potential pathogens to epithelial cells, thus exacerbating acute radiation-induced intestinal injury. This enables A. muciniphila to use mucin as an energy source. Additionally, A. muciniphila increases the inflammatory macrophage changes and secretion of inflammatory cytokines, leading to a decrease in epithelial stem cell density and inhibition of goblet cell differentiation, further exacerbating acute radiation-induced intestinal injury. Our findings suggest that in certain intestinal environments, the addition of A. muciniphila may worsen radiation-induced intestinal damage; thus, alternative approaches to reverse the dysbiosis associated with radiotherapy should be explored.
© The Author(s) 2025. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
-
Immunology and Microbiology
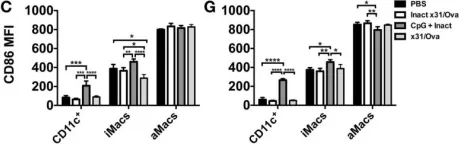
In Front Immunol on 15 July 2015 by Vogel, A. J. & Brown, D. M.
Fig.4.C,G

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 3
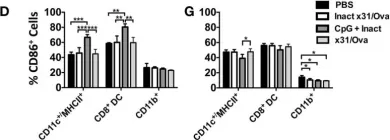
In Front Immunol on 15 July 2015 by Vogel, A. J. & Brown, D. M.
Fig.3.D,G

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 3
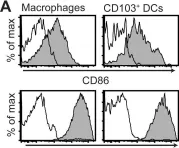
In PLoS One on 3 December 2010 by Quiding-Järbrink, M., Raghavan, S., et al.
Fig.3.A

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3