Density-based clustering is used in many contexts including in single molecule localization microscopy (SMLM), where it is commonly used to elucidate the nanoscale organization of molecules. However, little guidance is available for evaluating clustering performance, which is often dependent on user-input parameters. Here, we develop an efficient implementation of density-based cluster validation (DBCV) that can quantitatively evaluate clustering in SMLM-sized datasets. We demonstrate that maximizing DBCV scores accurately identifies clusters in noisy, simulated data. Coupling DBCV with Bayesian optimization, we outline a method, DBOpt, that selects input parameters in an unbiased manner for density-based clustering algorithms. We demonstrate that optimal parameters can be selected for popular algorithms (DBSCAN, HDBSCAN, OPTICS) with minimal user input. Finally, we show that DBOpt reports accurate feature sizes in 2D and 3D experimental data. In sum, DBOpt will improve the integrity, reproducibility, and quality of cluster analyses for SMLM and beyond.
© 2025. The Author(s).
Product Citations: 259
In Communications Biology on 10 June 2025 by Hammer, J. L., Devanny, A. J., et al.
Dynamic cytoskeletal regulation of cell shape supports resilience of lymphatic endothelium.
In Nature on 1 May 2025 by Schoofs, H., Daubel, N., et al.
Lymphatic capillaries continuously take up interstitial fluid and adapt to resulting changes in vessel calibre1-3. The mechanisms by which the permeable monolayer of loosely connected lymphatic endothelial cells (LECs)4 maintains mechanical stability remain elusive. Here we identify dynamic cytoskeletal regulation of LEC shape, induced by isotropic stretch, as crucial for the integrity and function of dermal lymphatic capillaries. We found that the oak leaf-shaped LECs showed a spectrum of VE-cadherin-based junctional configurations at the lobular intercellular interface and a unique cytoskeletal organization, with microtubules at concave regions and F-actin at convex lobes. Multispectral and longitudinal intravital imaging of capillary LEC shape and actin revealed dynamic remodelling of cellular overlaps in vivo during homeostasis and in response to interstitial fluid volume increase. Akin to puzzle cells of the plant epidermis5,6, LEC shape was controlled by Rho GTPase CDC42-regulated cytoskeletal dynamics, enhancing monolayer stability. Moreover, cyclic isotropic stretch increased cellular overlaps and junction curvature in primary LECs. Our findings indicate that capillary LEC shape results from continuous remodelling of cellular overlaps that maintain vessel integrity while preserving permeable cell-cell contacts compatible with vessel expansion and fluid uptake. We propose a bellows-like fluid propulsion mechanism, in which fluid-induced lumen expansion and shrinkage of LEC overlaps are countered by actin-based lamellipodia-like overlap extension to aid vessel constriction.
© 2025. The Author(s).
-
Cell Biology
Optogenetic control of mechanotransduction based on light-induced homodimerization of talin
Preprint on BioRxiv : the Preprint Server for Biology on 23 April 2025 by Nishimura, R., Barnett, S. F. H., et al.
Integrin-based cell adhesions (IACs) serve as primary sites where piconewton-scale actomyosin-generated mechanical forces are transmitted to the extracellular matrix (ECM), generating traction forces that drive cell-ECM responses including adhesion, migration, and mechano-signaling. Talin, a large (270 kDa) cytosolic adaptor protein, is the principal force-transmission protein in integrin-based adhesions, containing multiple mechanosensitive domains and protein-protein interaction sites that orchestrate molecular events in mechanosensing. As a highly modular multi-domain protein, talin has been identified as an effective target for chemogenetic and optogenetic manipulation of integrin-based mechanotransduction. However, a key limitation of previous approaches is the reliance on heterodimerization modules to control talin function, requiring the expression of two modified talin fragments. In practice, achieving precise expression levels in such a 2-component approach can be challenging, particularly when combined with other genetic tools. Since talin naturally contains a C-terminal dimerization domain that forms part of its actin-binding site, we reasoned that the molecularly engineered talin with a C-terminal optically-controlled homodimerizer could enable single-component optogenetic control of mechanotransduction. This approach would facilitate multiplexing with other molecular perturbations or experimental techniques. Here, we describe an opto-homodimerizable talin based on the pdDronpa1.2 optogenetic module, which enables optogenetic control of talin by a single construct. We demonstrate that light-induced talin dimerization promotes talin recruitment to IACs, adhesion formation, actin retrograde flow engagement, and downstream mechanotransduction signaling. Conversely, light-induced talin monomerization rapidly disassembles focal adhesions, disrupts talin-actin linkages, and accelerates actin retrograde flow, underscoring the critical roles of talin dimerization. Furthermore, our single-construct design allows facile multiplexing of optogenetic modulation of integrin-mediated mechanotransduction with super-resolution single-molecule tracking, revealing the essential role of talin dimerization for integrin α v β5 engagement.
Lysyl hydroxylase 2 glucosylates collagen VI to drive lung cancer progression.
In The Journal of Clinical Investigation on 1 April 2025 by Wang, S., Guo, H., et al.
Lysyl hydroxylase 2 (LH2) is highly expressed in multiple tumor types and accelerates disease progression by hydroxylating lysine residues on fibrillar collagen telopeptides to generate stable collagen cross links in tumor stroma. Here, we show that a galactosylhydroxylysyl glucosyltransferase (GGT) domain on LH2-modified type-VI collagen (Col6) to promote lung adenocarcinoma (LUAD) growth and metastasis. In tumors generated by LUAD cells lacking LH2 GGT domain activity, stroma was less stiff, and stable types of collagen cross links were reduced. Mass spectrometric analysis of total and glycosylated peptides in parental and GGT-inactive tumor samples identified Col6 chain α3 (Col6a3), a component of the Col6 heterotrimeric molecule, as a candidate LH2 substrate. In gain- and loss-of-function studies, high Col6a3 levels increased tumor growth and metastatic activity and enhanced the proliferative, migratory, and invasive activities of LUAD cells. LH2 coimmunoprecipitated with Col6a3, and LH2 glucosylated Col6 in an in vitro reaction. Glucosylation increased the integrin-binding and promigratory activities of Col6 in LUAD cells. Col6a3 K2049 was deglucosylated in GGT-inactive tumor samples, and mutagenesis of Col6a3 K2049 phenocopied Col6a3 deficiency or LH2 GGT domain inactivation in LUAD cells. Thus, LH2 glucosylates Col6 to drive LUAD progression. These findings show that the GGT domain of LH2 is protumorigenic, identify Col6 as a candidate effector, and provide a rationale to develop pharmacological strategies that target LH2's GGT domain in cancer cells.
-
Cancer Research
Coupling of cell shape, matrix and tissue dynamics ensures embryonic patterning robustness.
In Nature Cell Biology on 1 March 2025 by Moghe, P., Belousov, R., et al.
Tissue patterning coordinates morphogenesis, cell dynamics and fate specification. Understanding how precision in patterning is robustly achieved despite inherent developmental variability during mammalian embryogenesis remains a challenge. Here, based on cell dynamics quantification and simulation, we show how salt-and-pepper epiblast and primitive endoderm (PrE) cells pattern the inner cell mass of mouse blastocysts. Coupling cell fate and dynamics, PrE cells form apical polarity-dependent actin protrusions required for RAC1-dependent migration towards the surface of the fluid cavity, where PrE cells are trapped due to decreased tension. Concomitantly, PrE cells deposit an extracellular matrix gradient, presumably breaking the tissue-level symmetry and collectively guiding their own migration. Tissue size perturbations of mouse embryos and their comparison with monkey and human blastocysts further demonstrate that the fixed proportion of PrE/epiblast cells is optimal with respect to embryo size and tissue geometry and, despite variability, ensures patterning robustness during early mammalian development.
© 2025. The Author(s).
-
IHC-IF
-
Cell Biology
-
Stem Cells and Developmental Biology
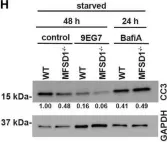
In Front Oncol on 26 February 2022 by Roblek, M., Bicher, J., et al.
Fig.5.H

-
WB
-
Collected and cropped from Front Oncol by CiteAb, provided under a CC-BY license
Image 1 of 5
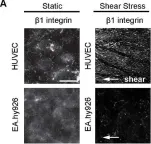
In Biol Open on 28 November 2014 by Macek Jílková, Z., Lisowska, J., et al.
Fig.2.A

-
ICC-IF
-
Collected and cropped from Biol Open by CiteAb, provided under a CC-BY license
Image 1 of 5
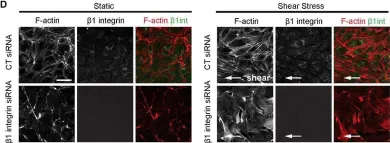
In Biol Open on 28 November 2014 by Macek Jílková, Z., Lisowska, J., et al.
Fig.2.D

-
IHC-IF
-
Collected and cropped from Biol Open by CiteAb, provided under a CC-BY license
Image 1 of 5
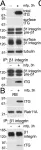
In PLoS One on 11 May 2011 by Zemskov, E. A., Mikhailenko, I., et al.
Fig.5.A,B

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 5
In PLoS One on 11 May 2011 by Zemskov, E. A., Mikhailenko, I., et al.
Fig.5.D,E,F

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 5