We describe the identification of a previously unrecognized ubiquitin ligase-substrate (FBXO45-GEF-H1) regulatory axis that plays an important role in germinal center formation and pathogenesis of common BCLs. These studies reveal novel insights linking dysregulated ubiquitin-mediated control to exploitable vulnerabilities and novel therapeutic strategies for these cancers.
©2025 The Authors; Published by the American Association for Cancer Research.
Product Citations: 99
The FBXO45-GEF-H1 Axis Controls Germinal Center Formation and B-cell Lymphomagenesis.
In Cancer Discovery on 2 April 2025 by Sahasrabuddhe, A. A., Chen, X., et al.
-
Cancer Research
-
Immunology and Microbiology
Minor Splicing Factor RNPC3 Is Essential for the Germinal Center B Cell Response.
In European Journal of Immunology on 1 April 2025 by Wang, J., Ruan, G. X., et al.
Germinal center (GC) response ensures the generation of diverse and high-affinity antibodies during the T cell-dependent (TD) immune response. This process is controlled by coordinated transcriptional and posttranscriptional gene regulatory mechanisms. Minor intron splicing is known to be involved in posttranscriptional regulation of gene expression. RNA-binding region (RNP1, RRM) containing 3 (RNPC3) is a minor spliceosome component involved in stabilizing the U11/U12 di-snRNP complex, which is essential for minor intron splicing. However, it remains unclear if RNPC3 and RNPC3-related gene regulatory mechanisms are important for the TD immune response. In this study, we conditionally ablated RNPC3 in activated B cells and showed that the mutant mice had defective antibody generation due to impaired GC B cell response. We demonstrate that RNPC3 deficiency inhibits the proliferation and promotes the apoptosis of activated B cells. Mechanistically, we show that RNPC3 regulates the development of GC B cells in a minor spliceosome-dependent manner by controlling the removal of minor introns from minor intron-containing genes associated with cell proliferation and apoptosis. Our study thus uncovers a previously unappreciated role for RNPC3 in regulating GC B cell response.
© 2025 Wiley‐VCH GmbH.
-
Immunology and Microbiology
In IScience on 18 October 2024 by Paradoski, B. T., Hou, S., et al.
B lymphocyte activation triggers metabolic reprogramming essential for B cell differentiation and mounting a healthy immune response. Here, we investigate the regulation and function of glucose-phosphorylating enzyme hexokinase 2 (HK2) in B cells. We report that both activation-dependent expression and mitochondrial localization of HK2 are regulated by the phosphatidylinositol 3-kinase (PI3K) signaling pathway. B cell-specific deletion of HK2 in mice caused mild perturbations in B cell development. HK2-deficient B cells show impaired functional responses in vitro and adapt to become less dependent on glucose and more dependent on glutamine. HK2 deficiency impairs glycolysis, alters metabolite profiles, and alters flux of labeled glucose carbons into downstream pathways. Upon immunization, HK2-deficient mice exhibit impaired germinal center, plasmablast, and antibody responses. HK2 expression in primary human chronic lymphocytic leukemia (CLL) cells was associated with recent proliferation and could be reduced by PI3K inhibition. Our study implicates PI3K-dependent modulation of HK2 in B cell metabolic reprogramming.
© 2024 The Author(s).
-
Biochemistry and Molecular biology
-
Cell Biology
Altered B Cell Metabolic Pathways Characterize Type 1 Diabetes Progression
Preprint on BioRxiv : the Preprint Server for Biology on 6 July 2024 by Conway, H., Perez, D., et al.
SUMMARY Type 1 diabetes (T1D) results in immune-mediated destruction of insulin-producing beta cells I the pancreas. B cells have been identified as critical antigen presenting cells and their specificity drives disease progression. More recently, they have also been shown to have the capacity to develop into suppressive, regulatory B cells that ameliorate autoimmune disease in animal models of rheumatoid arthritis and multiple sclerosis. In these models, signaling through hypoxia-inducible factor 1α (HIF-1α) drives a glycolytic flux that facilitates expansion of regulatory B cells. Here we examine the relationship between B cell development, cellular metabolism, and HIF-1α to reveal that in a mouse model of autoimmune diabetes, B cells have distinct metabolic characteristics that change with disease progression. Further, response to hypoxia in autoimmune B cells is distinct from the response by non-autoimmune control B cells. Together, these data suggest that dysregulated HIF signaling may drive T1D progression and activation of HIF-1α to expand regulatory B cell populations may be a viable option for immune modulation.
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 4 March 2024 by Paradoski, B. T., Hou, S., et al.
B lymphocyte metabolic reprogramming is essential for B cell differentiation and mounting a healthy immune response. The PI3K signaling pathway regulates B cell metabolism, but the mechanisms involved are not well understood. Here we report that signaling via PI3K8 can impact B cell glucose metabolism and immune functions via selective upregulation of hexokinase 2 (HK2). Three HK enzymes can catalyze the critical first step for glucose utilization and may selectively direct glucose into specific catabolic and anabolic pathways. While HK1 is constitutively expressed in B cells, HK2 is strikingly upregulated during B cell activation in a PI3K8-dependent manner. HK2 shows a unique distribution between mitochondrial and cytoplasmic pools that is also regulated by PI3K. Genetic deletion of HK2 significantly impairs extracellular acidification rate and glycolytic ATP production despite strong expression of HK1. B cell-specific deletion of HK2 in mice caused mild perturbations in B cell development but did not prevent generation of mature B cell subsets. HK2-deficient B cells show altered functional responses in vitro and evidence of metabolic adaptation to become less dependent on glucose and more dependent on glutamine. HK2-deficient B cells exhibit impaired glycolysis, altered metabolite profiles and altered flux of labeled glucose carbons into the pentose phosphate pathway. Upon immunization, HK2-deficient mice exhibit impaired generation of germinal centre B cells, plasmablasts and antibody responses. We further found that HK2 expression in primary human chronic lymphocytic leukemia (CLL) cells was associated with recent proliferation and could be reduced by PI3K inhibition. Our study identifies hexokinase 2 upregulation as a functionally important component of B cell metabolic reprogramming dependent on the PI3K pathway.
-
Biochemistry and Molecular biology
-
Cell Biology
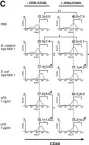
In BMC Immunol on 26 April 2012 by Gerlach, A. M., Steimle, A., et al.
Fig.2.A

-
FC/FACS
-
Collected and cropped from BMC Immunol by CiteAb, provided under a CC-BY license
Image 1 of 3
In BMC Immunol on 26 April 2012 by Gerlach, A. M., Steimle, A., et al.
Fig.2.C

-
FC/FACS
-
Collected and cropped from BMC Immunol by CiteAb, provided under a CC-BY license
Image 1 of 3
In BMC Immunol on 26 April 2012 by Gerlach, A. M., Steimle, A., et al.
Fig.2.B

-
FC/FACS
-
Collected and cropped from BMC Immunol by CiteAb, provided under a CC-BY license
Image 1 of 3