Preeclampsia is a leading cause of morbidity and mortality in pregnant women, affecting 5-8% of gestations worldwide. Its development is influenced by maternal immune abnormalities, metabolic disorders, and gut dysbiosis. In this study, we show that gut dysbiosis in pregnant C57BL/6J dams leads to increased fetal resorption, impaired placental development and altered vascularization. These adverse outcomes are associated with key pathological features of preeclampsia, including hypoxia, endoplasmic reticulum (ER) stress and reduction in uterine natural killer (NK) cell numbers. Furthermore, gut dysbiosis significantly perturbs placental carbohydrate metabolism, which impairs NK cell IFN-γ secretion. Notably, glucose supplementation restores placental NK cell function and reduces fetal resorption, suggesting that the observed impairment is reversible and dependent on a lower glycolytic rate. These findings highlight maternal gut microbiota as a key player in carbohydrate metabolism, with a pivotal role in modulating placental immunity and pregnancy outcome. The results provide valuable insights into potential metabolic biomarkers and suggest that targeting the gut microbiota may offer a strategy for preventing preeclampsia.
© 2025. The Author(s).
Product Citations: 255
In Nature Communications on 9 May 2025 by Giugliano, S., Gatti, A., et al.
-
Mus musculus (House mouse)
-
Endocrinology and Physiology
-
Immunology and Microbiology
In Cancer Metabolism on 26 November 2024 by Taş, İ., Varlı, M., et al.
Benzo[a]pyrene (BaP) is a toxic polycyclic aromatic hydrocarbon known as an exogenous AhR ligand. This study investigates the role of BaP in inducing immune checkpoint expression in lung adenocarcinoma (LUAD) and the underlying mechanisms involving the aryl hydrocarbon receptor (AhR) and tryptophan (Trp) metabolism.
We assessed the expression of immune checkpoint molecules, including PD-L1 and ICOSL, in lung epithelial cell lines (BEAS-2B and H1975) exposed to BaP. The involvement of AhR in BaP-induced immune checkpoint expression was examined using AhR silencing (siAhR). Additionally, the role of Trp metabolism in BaP-mediated immune evasion was explored through culturing in Trp (-/+) condition media, treatments with the inhibitors of rate-limiting enzymes in Trp metabolism (TDO2 and IDO1) and analyses of Trp-catabolizing enzymes. The therapeutic potential of targeting Trp metabolism, specifically TDO2, was evaluated in vivo using C57BL/6 mice orthotopically inoculated with LUAD cells.
BaP exposure significantly upregulated the mRNA and surface expression of PD-L1 and ICOSL, with AhR playing a crucial role in this induction. Trp metabolism was found to enhance BaP-mediated immune evasion, as indicated by stronger induction of immune checkpoints in Trp (+) media and the upregulation of Trp-catabolizing enzymes. TDO2 inhibition markedly suppressed the surface expression of PD-L1 and ICOSL, demonstrating the importance of Trp metabolism in BaP-induced immune evasion. Further analysis confirmed the high TDO2 expression in lung adenocarcinoma and its association with poor patient survival. Using an orthotopic implantation mouse model, we demonstrated the inhibitory effect of two different TDO2 inhibitors on tumorigenesis, immune checkpoints, and tryptophan metabolism.
This study highlights the key mechanisms behind BaP-induced immune evasion in LUAD, particularly through the TDO2/AhR axis. It reveals how TDO2 inhibitors can counteract immune checkpoint activation and boost anti-tumor immunity, suggesting new paths for targeted lung cancer immunotherapy. The findings significantly improve our understanding of immune evasion in LUAD and underscore the therapeutic promise of TDO2 inhibition.
© 2024. The Author(s).
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
HDAC6 Deletion Decreases Pristane-induced Inflammation.
In ImmunoHorizons on 1 September 2024 by Xu, D., Luo, X. M., et al.
Systemic lupus erythematosus is an autoimmune disease characterized by excessive inflammation and production of pathogenic Abs. Histone deacetylase 6 (HDAC6) is a class IIb histone deacetylase. It has been reported that selective HDAC6 inhibition decreases inflammation in lupus mouse models. In this study, sex- and age-matched wild-type (WT) and HDAC6-/- mice on the C57BL/6 background were administered 0.5 ml of pristane or PBS i.p. at 8-12 wk of age and were euthanized 10 d later. At sacrifice, body weight and spleen weight were measured, sera were collected, and splenocytes and peritoneal cells were harvested for flow cytometry. We found pristane administration increased the spleen weight with no difference between WT and HDAC6-/- mice. Pristane administration promoted the population of CD11b+Ly6C++ inflammatory monocytes and CD11b+Ly6G+ neutrophils. Peritoneal recruitment of these inflammatory monocytes and neutrophils was significantly decreased in HDAC6-/- mice compared with the WT mice. Flow cytometry results showed that the number of CD69+ T and B cells was increased in HDAC6-/- mice. Pristane administration also induced the IFN signature genes as determined by RT-qPCR. Furthermore, IFN signature genes were not affected in HDAC6-/- mice compared with the WT mice. In vitro studies in J774A.1 cells revealed that the selective HDAC6 inhibitor (ACY-738) increased acetylation of NF-κB while increasing Stat1 phosphorylation, which resulted in inducible NO synthase production in LPS/IFN-γ-stimulated cells. Taken together, these results demonstrate that although HDAC6 inhibition may inhibit some inflammatory pathways, others remain unaffected.
Copyright © 2024 The Authors.
-
Immunology and Microbiology
Enrichment of liver MAIT cells in a mouse model of Alzheimer's disease.
In Journal of Neuroimmunology on 15 May 2024 by Wyatt-Johnson, S. K., Kersey, H. N., et al.
Emerging evidence has supported a role for the immune system and liver in Alzheimer's disease (AD). However, our understanding of how hepatic immune cells are altered in AD is limited. We previously found that brain mucosal-associated invariant T (MAIT) cell numbers are increased in AD. Furthermore, loss of MAIT cells and their antigen-presenting molecule, MR1, reduced amyloid-β accumulation in the brain. MAIT cells are also significantly present in the liver. Therefore, we sought to analyze MAIT and other immune cells in the AD liver. Increased frequency of activated MAIT cells (but not conventional T cells) were found in 8-month-old 5XFAD mouse livers. Therefore, these data raise the possibility that there is a role for peripheral MAIT cells in AD pathology.
Copyright © 2024 Elsevier B.V. All rights reserved.
-
Immunology and Microbiology
-
Neuroscience
In Nature Communications on 12 April 2024 by Barnkob, M. B., Michaels, Y. S., et al.
Semaphorin-3A (SEMA3A) functions as a chemorepulsive signal during development and can affect T cells by altering their filamentous actin (F-actin) cytoskeleton. The exact extent of these effects on tumour-specific T cells are not completely understood. Here we demonstrate that Neuropilin-1 (NRP1) and Plexin-A1 and Plexin-A4 are upregulated on stimulated CD8+ T cells, allowing tumour-derived SEMA3A to inhibit T cell migration and assembly of the immunological synapse. Deletion of NRP1 in both CD4+ and CD8+ T cells enhance CD8+ T-cell infiltration into tumours and restricted tumour growth in animal models. Conversely, over-expression of SEMA3A inhibit CD8+ T-cell infiltration. We further show that SEMA3A affects CD8+ T cell F-actin, leading to inhibition of immune synapse formation and motility. Examining a clear cell renal cell carcinoma patient cohort, we find that SEMA3A expression is associated with reduced survival, and that T-cells appear trapped in SEMA3A rich regions. Our study establishes SEMA3A as an inhibitor of effector CD8+ T cell tumour infiltration, suggesting that blocking NRP1 could improve T cell function in tumours.
© 2024. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
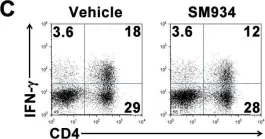
In PLoS One on 6 March 2012 by Hou, L. F., He, S. J., et al.
Fig.6.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2
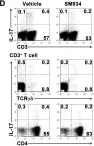
In PLoS One on 6 March 2012 by Hou, L. F., He, S. J., et al.
Fig.6.D

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2