The liver lymphatic system plays a critical role in maintaining interstitial fluid balance and immune regulation. Efficient lymphatic drainage is essential for liver homeostasis, but its role in liver disease progression remains poorly understood. In cirrhosis, lymphangiogenesis initially compensates for increased lymph production, but impaired lymphatic drainage in advanced stages may lead to complications such as ascites and portal hypertension. This study aimed to evaluate how liver lymphatic dysfunction affects disease progression and to assess therapeutic strategies. Using a surgical model to block liver lymphatic outflow, we found that impaired drainage accelerates liver injury, fibrosis, and immune cell infiltration, even in healthy livers. Mechanistically, enhanced TGF-β signaling in liver lymphatic endothelial cells (LyECs) contributed to reduced lymphatic vessel (LV) density and function in late-stage decompensated cirrhosis. This dysfunction was linked to the progression from compensated to decompensated cirrhosis, particularly in patients with primary sclerosing cholangitis (PSC). Conversely, liver-specific overexpression of VEGF-C via AAV8 improved lymphatic drainage, restored LV density, reduced fibrosis, mitigated liver injury, and alleviated portal hypertension in cirrhotic rats. These findings establish impaired liver lymphatic function as a pivotal driver of cirrhosis progression and identify VEGF-C as a promising therapeutic target to prevent decompensation.
Product Citations: 145
Liver Lymphatic Dysfunction as a Driver of Fibrosis and Cirrhosis Progression
Preprint on BioRxiv : the Preprint Server for Biology on 14 January 2025 by Jeong, J., Hsu, S., et al.
-
Mus musculus (House mouse)
In Journal of Translational Medicine on 6 November 2024 by García-Martín, A., Prados, M. E., et al.
Vasculogenic therapies explored for the treatment of peripheral artery disease (PAD) have encountered minimal success in clinical trials. Addressing this, B55α, an isoform of protein phosphatase 2A (PP2A), emerges as pivotal in vessel remodeling through activation of hypoxia-inducible factor 1α (HIF-1α). This study delves into the pharmacological profile of VCE-004.8 (Etrinabdione) and evaluates its efficacy in a preclinical model of critical limb ischemia, with a focus on its potential as a PP2A/B55α activator to induce angiogenesis and arteriogenesis.
Vascular endothelial cells were used for in vitro experiments. Aorta ring assay was performed to explore sprouting activity. Matrigel plug-in assay was used to assess the angiogenic potential. Critical limb ischemia (CLI) in mice was induced by double ligation in the femoral arteria. Endothelial vascular and fibrotic biomarkers were studied by immunohistochemistry and qPCR. Arteriogenesis was investigated by microvascular casting and micro-CT. Proteomic analysis in vascular tissues was analyzed by LC-MS/MS. Ex-vivo expression of B55α and biomarkers were investigated in artery samples from PAD patients.
VCE-004.8 exhibited the ability to induce B55α expression and activate the intersecting pathways B55α/AMPK/Sirtuin 1/eNOS and B55α/PHD2/HIF-1α. VCE-004.8 prevented OxLDL and H2O2-induced cytotoxicity, senescence, and inflammation in endothelial cells. Oral VCE-004.8 increased aorta sprouting in vitro and angiogenesis in vivo. In CLI mice VCE-004.8 improved collateral vessel formation and induced endothelial cells proliferation, angiogenic gene expression and prevented fibrosis. The expression of B55α, Caveolin 1 and Sirtuin-1 is reduced in arteries from CLI mice and PAD patient, and the expression of these markers was restored in mice treated with VCE-004.8.
The findings presented in this study indicate that Etrinabdione holds promise in mitigating endothelial cell damage and senescence, while concurrently fostering arteriogenesis and angiogenesis. These observations position Etrinabdione as a compelling candidate for the treatment of PAD, and potentially other cardiovascular disorders.
© 2024. The Author(s).
-
Mus musculus (House mouse)
In International Journal of Stem Cells on 23 October 2024 by Kang, G. H., Shin, Y. K., et al.
Stem cells derived from human orbicularis oculi muscle (hOOM) are a valuable resource for cell therapy. However, when stem cells are continuously cultured, their abilities tend to deteriorate over time. One method to address this issue is to use basic fibroblast growth factor (bFGF) to maintain the stem cell functionality. The limitation is that bFGF is unstable under mammalian cell culture conditions with a half-life of only 8 hours, which poses a significant challenge to the production and maintenance of high-quality stem cells. In this study, we used thermostable bFGF (TS-bFGF) and demonstrated that hOOM-derived stem cells cultured with TS-bFGF exhibited superior proliferation, stem cell function, reduced reactive oxygen species, and cellular senescence delay effect compared to cells cultured with wild-type bFGF. Considering the pivotal role of stem cells in broad ranges of applications such as regenerative medicine and cultured meat, we anticipate that TS-bFGF, owing to its thermostability and long-lasting properties, will contribute significantly to the acquisition of high-quality stem cells.
-
FC/FACS
-
Stem Cells and Developmental Biology
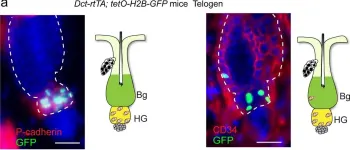
Transcription factor KROX20 marks epithelial stem cells for hair follicle formation.
In The Journal of Clinical Investigation on 3 October 2024 by Ghotbi, E., Tchegnon, E., et al.
Epidermal stem cells control homeostasis and regeneration of skin and hair. In the hair follicle (HF) bulge of mammals, populations of slow-cycling stem cells regenerate the HF during cyclical rounds of anagen (growth), telogen (quiescence), and catagen (regression). Multipotent epidermal cells are also present in the HF above the bulge area, contributing to the formation and maintenance of sebaceous gland and upper and middle portions of the HF. Here, we report that the transcription factor Krox20 is enriched in an epidermal stem cell population located in the upper/ middle HF. Expression analyses and lineage tracing using inducible Krox20-CreERT showed that Krox20-lineage cells migrate out of this HF region and contribute to the formation of bulge in the HF, serving as ancestors of bulge stem cells. In vivo depletion of these cells arrests HF morphogenesis. This study identifies a novel marker for an epidermal stem cell population that is indispensable for hair homeostasis.
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Stem Cells and Developmental Biology
In Nature Communications on 12 March 2024 by Martins, L. R., Sieverling, L., et al.
Understanding the molecular and cellular processes involved in lung epithelial regeneration may fuel the development of therapeutic approaches for lung diseases. We combine mouse models allowing diphtheria toxin-mediated damage of specific epithelial cell types and parallel GFP-labeling of functionally dividing cells with single-cell transcriptomics to characterize the regeneration of the distal lung. We uncover cell types, including Krt13+ basal and Krt15+ club cells, detect an intermediate cell state between basal and goblet cells, reveal goblet cells as actively dividing progenitor cells, and provide evidence that adventitial fibroblasts act as supporting cells in epithelial regeneration. We also show that diphtheria toxin-expressing cells can persist in the lung, express specific inflammatory factors, and transcriptionally resemble a previously undescribed population in the lungs of COVID-19 patients. Our study provides a comprehensive single-cell atlas of the distal lung that characterizes early transcriptional and cellular responses to concise epithelial injury, encompassing proliferation, differentiation, and cell-to-cell interactions.
© 2024. The Author(s).
-
Mus musculus (House mouse)
In Nature on 1 April 2023 by Sun, Q., Lee, W., et al.
Fig.1.A

-
IHC-IF
-
Collected and cropped from Nature by CiteAb, provided under a CC-BY license
Image 1 of 1