Despite major therapeutic advances in the treatment of acute lymphoblastic leukemia (ALL), resistances and long-term toxicities still pose significant challenges. Cyclins and their associated cyclin-dependent kinases are one focus of cancer research when looking for targeted therapies. We discovered cyclin C to be a key factor for B-cell ALL (B-ALL) development and maintenance. While cyclin C is not essential for normal hematopoiesis, CcncΔ/Δ BCR::ABL1+ B-ALL cells fail to elicit leukemia in mice. RNA sequencing experiments revealed a p53 pathway deregulation in CcncΔ/Δ BCR::ABL1+ cells resulting in the inability of the leukemic cells to adequately respond to stress. A genome-wide CRISPR/Cas9 loss-of-function screen supplemented with additional knock-outs unveiled a dependency of human B-lymphoid cell lines on CCNC. High cyclin C levels in B-cell precursor (BCP) ALL patients were associated with poor event-free survival and increased risk of early disease recurrence after remission. Our findings highlight cyclin C as a potential therapeutic target for B-ALL, particularly to enhance cancer cell sensitivity to stress and chemotherapy.
Product Citations: 134
In Haematologica on 1 April 2025 by Trifinopoulos, J., List, J., et al.
-
Mus musculus (House mouse)
-
Cancer Research
-
Cardiovascular biology
-
Immunology and Microbiology
In The Journal of Clinical Investigation on 21 January 2025 by Cui, X., Hou, L., et al.
The bone marrow (BM) niche is critical in regulating hematopoiesis, and sexual dimorphism and its underlying mechanism in the BM niche and its impact on hematopoiesis are not well understood. We show that male mice exhibited a higher abundance of leptin-receptor-expressing mesenchymal stromal cells (LepR-MSCs) compared with female mice. Sex-mismatched coculture and BM transplantation showed that the male BM niche provided superior support for in vitro colony formation and in vivo hematopoietic engraftment. The cotransplantation of male stromal cells significantly enhanced engraftment in female recipients. Single-cell RNA-seq revealed that the lower expression of the X-linked lysine H3K4 demethylase, Kdm5c, in male MSCs led to the increased expression of Cxcl12. In MSC-specific Kdm5c-KO mouse model, the reduction of KDM5C in female MSCs enhanced MSC quantity and function, ultimately improving engraftment to the male level. Kdm5c thus plays a role in driving sexual dimorphism in the BM niche and hematopoietic regeneration. Our study unveils a sex-dependent mechanism governing the BM niche regulation and its impact on hematopoietic engraftment. The finding offers potential implications for enhancing BM transplantation efficacy in clinical settings by harnessing the resource of male MSCs or targeting Kdm5c.
Preprint on Research Square on 11 September 2023 by Shen, X., Fu, D., et al.
Backgroud: During various stages of fracture healing, macrophages control mesenchymal stem cells' (BMSCs') proliferative behavior and osteogenic differentiation through varying polarization states. BMSCs also regulate their own osteogenic differentiation through the polarization state of macrophages to meet the requirements of tissue repair and osteogenic environment. A crucial role in cell proliferation, differentiation, and death is played by the evolutionarily conserved Notch signaling system. It also plays an important role in the osteogenic differentiation and regulation of macrophage polarization of BMSCs. The NOTCH signaling pathway typically plays a role in information exchange through direct contact between cells. Therefore, the Notch signaling pathway is involved in information exchange during direct contact between macrophages and BMSCs. Methods: A co culture system of mouse monocytic megacytic leukemia cell line (RAW264.7) and BMSC was established. RAW264.7 cells in logarithmic growth phase were divided into M0 group (unpolarized) and M1 group (LPS+INF γ induction), M2 group (IL4+IL13 induction), polarization status was detected by flow cytometry, and then BMSC were added to detect the Notch signaling pathway and the expression levels of RUNX2 gene and protein at different time points in each group. To further validate the role of the NOTCH signaling pathway in osteogenesis, we chose to apply the NOTCH signaling blocker RO4929097 to the co culture system of M2 and BMSC.According to whether blockers were used or not, they were divided into control group, M2 group, M2+blocker group, and blocker group. The transmission of the NOTCH signaling pathway in the interaction between M2 and BMSC as well as the production of Hes1 linked to the osteogenic gene RUNX2 were observed by blocking the NOTCH signaling pathway's conduction. At the same time, we detected the polarization of RAW264.7 cells in Mo and M1 groups to determine whether there was a change in the polarization state of RAW264.7 cells after the addition of BMSC. Results: : PCR and WB results showed that the NOTCH signaling pathway and osteogenic specific RUNX2 related protein and gene expression were basically synchronized: the expression of Jagged1 and Notch1 in M2 group was higher than that in M0 and M1 groups (p<0.05), while the expression level of M0 group was higher than that in M1 group (p<0.05). Hes1, as an associated gene and protein of Notch signaling pathway and Runx2, had the highest expression level with RUNX2 in M2 group (p<0.05), followed by M0, and the lowest in M1 group.This revealed that the Notch signaling pathway is involved in the bone immune regulatory effect between RAW264.7 and BMSC. After administering the NOTCH signaling blocker RO4929097, the M2 group had the highest expression of Notch signaling pathway related protein genes (p<0.05), followed by the control group (<0.05), and the blocker group had the lowest expression level (p<0.05), indicating a higher expression of the NOTCH signaling pathway between M2 cells and BMSC. The M2+blocker group had a higher expression level than the blocker group, suggesting that there are other pathways between M2 and BMSC that affect the conduction of the NOTCH signaling pathway. BMSC and RAW264.7 were co cultured, and flow cytometry analysis showed that the proportion of M2 like cells in the M0 group was higher than that in the M1 group. Conclusion: In the co culture system of macrophages and BMSC,the Notch signaling pathway promotes macrophage polarization towards M2 type, thereby regulating the osteogenic differentiation of BMSC and participating in the bone immune regulation of macrophages and mesenchymal stem cells.
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
In Nature Cancer on 1 August 2023 by Grockowiak, E., Korn, C., et al.
Aging facilitates the expansion of hematopoietic stem cells (HSCs) carrying clonal hematopoiesis-related somatic mutations and the development of myeloid malignancies, such as myeloproliferative neoplasms (MPNs). While cooperating mutations can cause transformation, it is unclear whether distinct bone marrow (BM) HSC-niches can influence the growth and therapy response of HSCs carrying the same oncogenic driver. Here we found different BM niches for HSCs in MPN subtypes. JAK-STAT signaling differentially regulates CDC42-dependent HSC polarity, niche interaction and mutant cell expansion. Asymmetric HSC distribution causes differential BM niche remodeling: sinusoidal dilation in polycythemia vera and endosteal niche expansion in essential thrombocythemia. MPN development accelerates in a prematurely aged BM microenvironment, suggesting that the specialized niche can modulate mutant cell expansion. Finally, dissimilar HSC-niche interactions underpin variable clinical response to JAK inhibitor. Therefore, HSC-niche interactions influence the expansion rate and therapy response of cells carrying the same clonal hematopoiesis oncogenic driver.
© 2023. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Stem Cells and Developmental Biology
In Neural Regeneration Research on 1 May 2023 by Sun, X., Huang, L. Y., et al.
Although many therapeutic interventions have shown promise in treating spinal cord injury, focusing on a single aspect of repair cannot achieve successful and functional regeneration in patients following spinal cord injury . In this study, we applied a combinatorial approach for treating spinal cord injury involving neuroprotection and rehabilitation, exploiting cell transplantation and functional sensorimotor training to promote nerve regeneration and functional recovery. Here, we used a mouse model of thoracic contusive spinal cord injury to investigate whether the combination of bone marrow mesenchymal stem cell transplantation and exercise training has a synergistic effect on functional restoration. Locomotor function was evaluated by the Basso Mouse Scale, horizontal ladder test, and footprint analysis. Magnetic resonance imaging, histological examination, transmission electron microscopy observation, immunofluorescence staining, and western blotting were performed 8 weeks after spinal cord injury to further explore the potential mechanism behind the synergistic repair effect. In vivo, the combination of bone marrow mesenchymal stem cell transplantation and exercise showed a better therapeutic effect on motor function than the single treatments. Further investigations revealed that the combination of bone marrow mesenchymal stem cell transplantation and exercise markedly reduced fibrotic scar tissue, protected neurons, and promoted axon and myelin protection. Additionally, the synergistic effects of bone marrow mesenchymal stem cell transplantation and exercise on spinal cord injury recovery occurred via the PI3K/AKT/mTOR pathway. In vitro, experimental evidence from the PC12 cell line and primary cortical neuron culture also demonstrated that blocking of the PI3K/AKT/mTOR pathway would aggravate neuronal damage. Thus, bone marrow mesenchymal stem cell transplantation combined with exercise training can effectively restore motor function after spinal cord injury by activating the PI3K/AKT/mTOR pathway.
-
FC/FACS
-
Mus musculus (House mouse)
-
Neuroscience
-
Stem Cells and Developmental Biology
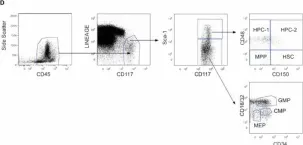
In Elife on 20 September 2022 by Boroumand, P., Prescott, D. C., et al.
Fig.2.D

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 2
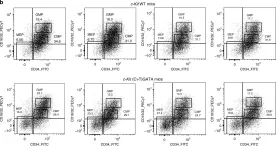
In Nat Commun on 22 May 2020 by Yang, L., Chen, Z., et al.
Fig.5.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 2