Platelets, known for maintaining blood balance, also participate in antimicrobial defense. Upon severeacute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, platelets become hyperactivated, releasing molecules such as cytokines, granule contents, and bioactive lipids. The key effector biolipids produced by platelets include 12-hydroxyeicosatetraenoic acid (12-HETE) and 12-hydroxyeicosatrienoic acid (12-HETrE), produced by 12-lipoxygenase (12-LOX), and prostaglandins and thromboxane, produced by cyclooxygenase-1. While prostaglandin E2 and thromboxane B2 were previously associated with lung inflammation in severe COVID-19, the role of platelet 12-LOX in SARS-CoV-2 infection remains unclear. Using mice deficient for platelets' 12-LOX, we report that SARS-CoV-2 infection resulted in higher lung inflammation characterized by histopathological tissue analysis, increased leukocyte infiltrates, and cytokine production relative to wild-type mice. In addition, distinct platelet and lung transcriptomic changes, including alterations in NOD-like receptor (NLR) family pyrin domain-containing 1 (NLRP1) inflammasome-related gene expression, were observed. Mass spectrometry lipidomic analysis in 12-LOX-deficient-infected mice revealed significant changes in bioactive lipid content, including reduced levels of 12-HETrE that inversely correlated with disease severity. Finally, platelet 12-LOX deficiency was associated with increased morbidity and lower survival rates relative to wild type (WT) mice. Overall, this study highlights the complex interplay between 12-LOX-related lipid metabolism and inflammatory responses during SARS-CoV-2 infection. The findings provide valuable insights into potential therapeutic targets aimed at mitigating severe outcomes, emphasizing the pivotal role of platelet enzymes in the host response to viral infections.
Product Citations: 53
In Proceedings of the National Academy of Sciences of the United States of America on 25 March 2025 by Dos S P Andrade, A. C., Lacasse, E., et al.
-
COVID-19
-
Immunology and Microbiology
In Genes on 6 March 2025 by Askari, S. & Goldfinger, L. E.
Platelets are highly enriched in microRNAs (miRNAs), which are genomically encoded 19-25 nucleotide non-coding RNAs that target complementary mRNAs through total or near-total base pairing. MiR-223 is among the most abundant miRNAs in human and murine platelets, but despite ongoing investigations in recent years, miR-223 roles in platelet physiology and its putative roles in high on-treatment platelet reactivity (HTPR) remain controversial, as studies showed varying findings.
In the current hybrid review/report, we aim to compare studies that investigated miR-223 in platelet function and HTPR. Additionally, we briefly report our own findings on murine miR-223-deficient platelets.
We have thoroughly searched the literature and found three studies that investigated the roles of miR-223 in platelet function by utilizing miR-223 global knockout mice, and three studies that explored the association between miR-223 and residual platelet reactivity by measuring miR-223 levels in platelets of patients treated with clopidogrel for cardiac artery disease. We assessed platelet function in response to different agonists and evaluated P2y12 levels in male and female miR-223-deficient platelets.
Integrin activation and α granule secretion were similar between WT and KO platelets in response to all agonists in platelets from both female and male mice, although both genotypes showed elevated thrombin response in females compared to males.
In all studies, including ours, taken together, miR-233 appears to play a modest role in platelet function and development of HTPR.
-
Genetics
Kindlin-3 phosphorylation is crucial for thrombosis and hemostasis in vivo.
In Research and Practice in Thrombosis and Haemostasis on 1 March 2025 by Pluskota, E., Szpak, D., et al.
A single phosphorylation in kindlin-3 at S484 (human) or S485 (mouse) has been shown to regulate its function in a variety of cells in vitro. However, whether this posttranslational modification of kindlin-3 plays a role in platelets and in hemostasis and thrombosis in vivo remains totally unknown.
To create a strain of mice expressing kindlin-3 that bears the S485A substitution and utilize these mice to determine if kindlin-3 phosphorylation influences platelet responses, hemostasis, and thrombosis in vivo.
By clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 gene editing, we generated a mouse strain that expressed a kindlin-3 mutant bearing the S485A substitution. S485A kindlin-3 mice were born normally, and the platelet and red blood cells numbers were similar to their wild-type counterparts. Wild-type and S485A kindlin-3 platelets showed similar expression of αIIbβ3 integrin, kindlin-3, and talin.
Platelets isolated from S485A kindlin-3 mice did not undergo agonist-induced kindlin-3 phosphorylation. Functional analysis revealed that S485A kindlin-3 mice exhibited prolonged tail bleeding time, increased blood loss, and delayed thrombosis in the carotid artery injury model in vivo. Platelets isolated from S485A kindlin-3 mice showed defective platelet function, including impaired integrin αIIbβ3 activation, platelet aggregation, clot retraction, and adhesion.
Our observations demonstrate that kindlin-3 phosphorylation on S485 in mice is crucial for supporting activation of αIIbβ3 integrin and αIIbβ3 integrin-dependent platelet responses and consequently contributes to hemostasis and thrombosis in vivo.
© 2025 The Authors.
In Scientific Reports on 28 January 2025 by Lazar, S., Wurtzel, J. G. T., et al.
Platelets are enriched in miRNAs and harbor Ago2 as the principal RNA silencing Argonaute. However, roles in thrombopoiesis and platelet function remain poorly understood. We generated megakaryocyte/platelet-specific Ago2-deleted (Ago2 KO) mice and assessed proteomic and functional effects. We predicted platelet hyperreactivity with Ago2 deletion due to large-scale upregulated protein expression. Platelet counts were normal. Mean volumes were increased, associated with larger, though fewer megakaryocytes. Ago2-deleted platelets from male mice showed hyperreactivity to thromboxane but not to other agonists compared to controls, whereas Ago2-deleted platelets from female mice showed normal reactivity. Ago2 KO mice displayed normal hemostasis and clot dynamics. Proteomes of Ago2-deleted and wild type platelets were mostly similar. However, Ago1 - undetectable in wild type platelets - was upregulated in Ago2-deleted platelets in both males and females, confirmed by immunoblotting. Female Ago2-deleted platelets selectively showed downregulation of a protein cohort established in breast cancer cells to be transcriptionally regulated by estrogen receptor-beta coupled to Ago2, whereas male Ago2-deleted platelets did not. Thus, Ago2 is important for platelet development and function, putatively partially rescued by upregulation of Ago1. Platelet reactivity controlled by Ago2 reflects sex-specific regulation of gene expression potentially at both transcriptional and translational levels in megakaryocytes and platelets.
© 2025. The Author(s).
-
Mus musculus (House mouse)
In Stem Cell Research & Therapy on 29 April 2024 by Vercellino, J., Małachowska, B., et al.
Acute radiation syndrome (ARS) manifests after exposure to high doses of radiation in the instances of radiologic accidents or incidents. Facilitating regeneration of the bone marrow (BM), namely the hematopoietic stem and progenitor cells (HSPCs), is key in mitigating ARS and multi-organ failure. JNJ-26366821, a PEGylated thrombopoietin mimetic (TPOm) peptide, has been shown as an effective medical countermeasure (MCM) to treat hematopoietic-ARS (H-ARS) in mice. However, the activity of TPOm on regulating BM vascular and stromal niches to support HSPC regeneration has yet to be elucidated.
C57BL/6J mice (9-14 weeks old) received sublethal or lethal total body irradiation (TBI), a model for H-ARS, by 137Cs or X-rays. At 24 h post-irradiation, mice were subcutaneously injected with a single dose of TPOm (0.3 mg/kg or 1.0 mg/kg) or PBS (vehicle). At homeostasis and on days 4, 7, 10, 14, 18, and 21 post-TBI with and without TPOm treatment, BM was harvested for histology, BM flow cytometry of HSPCs, endothelial (EC) and mesenchymal stromal cells (MSC), and whole-mount confocal microscopy. For survival, irradiated mice were monitored and weighed for 30 days. Lastly, BM triple negative cells (TNC; CD45-, TER-119-, CD31-) were sorted for single-cell RNA-sequencing to examine transcriptomics after TBI with or without TPOm treatment.
At homeostasis, TPOm expanded the number of circulating platelets and HSPCs, ECs, and MSCs in the BM. Following sublethal TBI, TPOm improved BM architecture and promoted recovery of HSPCs, ECs, and MSCs. Furthermore, TPOm elevated VEGF-C levels in normal and irradiated mice. Following lethal irradiation, mice improved body weight recovery and 30-day survival when treated with TPOm after 137Cs and X-ray exposure. Additionally, TPOm reduced vascular dilation and permeability. Finally, single-cell RNA-seq analysis indicated that TPOm increased the expression of collagens in MSCs to enhance their interaction with other progenitors in BM and upregulated the regeneration pathway in MSCs.
TPOm interacts with BM vascular and stromal niches to locally support hematopoietic reconstitution and systemically improve survival in mice after TBI. Therefore, this work warrants the development of TPOm as a potent radiation MCM for the treatment of ARS.
© 2024. The Author(s).
-
Stem Cells and Developmental Biology
In Front Neurosci on 21 March 2020 by Kniewallner, K. M., de Sousa, D. M. B., et al.
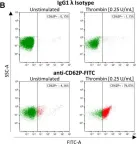
Fig.2.B
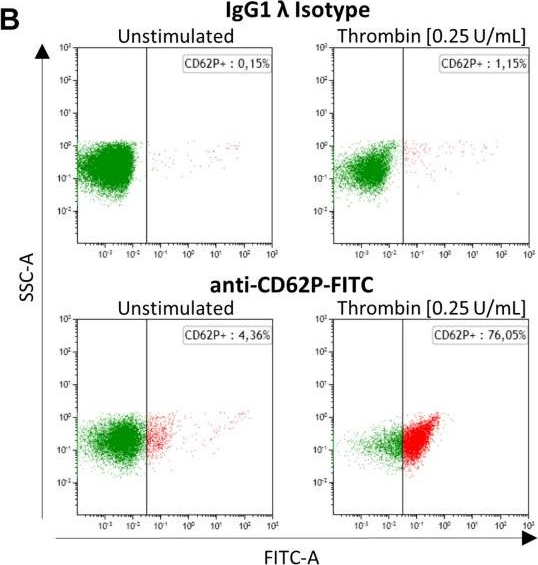
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Frontiers in Neuroscience by CiteAb, provided under a CC-BY license
Image 1 of 2
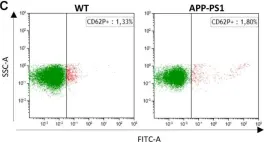
In Front Neurosci on 21 March 2020 by Kniewallner, K. M., de Sousa, D. M. B., et al.
Fig.2.C
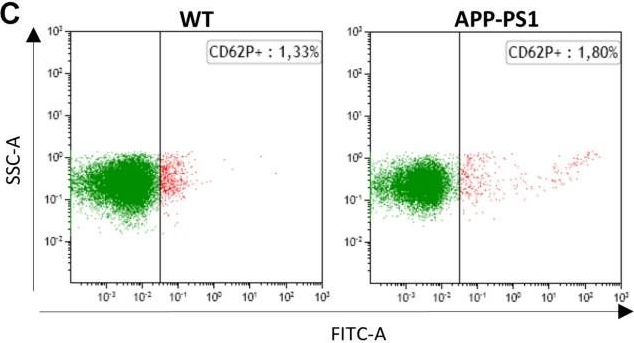
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Frontiers in Neuroscience by CiteAb, provided under a CC-BY license
Image 1 of 2