Although imiquimod (IMQ) is widely used to induce psoriasis-like inflammation in mouse models, its direct effects on dendritic cells (DCs) and their capacity to drive T cell polarization remain poorly defined. The systemic complexity of in vivo models hinders the ability to delineate the direct, cell-intrinsic effects of IMQ. To address this gap, an in vitro DC-CD4+ T cell co-culture system was established using bone marrow-derived DCs and naïve CD4+ T cells isolated from OT-II mice, enabling precise evaluation of the direct immunomodulatory effects of IMQ. IMQ treatment markedly upregulated maturation markers (CD40, CD80, CD86 and major histocompatibility complex class II), and increased IL-12 and IL-6 secretion in a dose-dependent manner. These effects were significantly attenuated by NF-κB inhibitors (caffeic acid phenethyl ester and Bay 11-7082), indicating a critical role for NF-κB signaling in DC activation. When co-cultured with naïve CD4+ T cells, IMQ-treated DCs promoted robust differentiation toward T helper (Th)1 and Th17 subsets. Neutralization of IL-12 and IL-6 in the co-culture system significantly reduced the frequencies of Th1 and Th17 cells and their cytokine output, confirming that these responses were mediated by DC-derived cytokines. Collectively, the present findings demonstrated that IMQ directly activated DCs via NF-κB signaling and induced pathogenic Th cell responses through IL-12 and IL-6 production. By eliminating confounding in vivo factors, the present study provided evidence for the DC-intrinsic effects of IMQ and offered mechanistic insights into the cellular pathways linking innate immune sensing to adaptive T cell responses in psoriasis.
Copyright © 2025, Spandidos Publications.
Product Citations: 139
In Experimental and Therapeutic Medicine on 1 September 2025 by Cho, Y., Kwon, J., et al.
In Cell Biology and Toxicology on 8 March 2025 by Dong, X., Wang, X., et al.
Triple-negative breast cancer (TNBC) poses as a daunting and intricate manifestation of breast cancer, highlighted by few treatment options and a poor outlook. The crucial element in fostering tumor growth and immune resistance is the polarization of tumor-associated macrophages (TAMs) into the M2 state within the tumor microenvironment (TME). To address this, we developed M2 targeting peptide-chitosan-curcumin nanoparticles (M2pep-Cs-Cur NPs), a targeted delivery system utilizing chitosan (Cs) as a carrier, curcumin (Cur) as a therapeutic agent, and targeting peptides for specificity. These NPs effectively inhibited TNBC cell proliferation (~ 70%) and invasion (~ 70%), while increasing the responsiveness of tumors to anti-PD-L1 treatment (~ 50% survival enhancement) in vitro and in vivo. Bioinformatics analysis suggested that Cur modulates TAM polarization by influencing key genes such as COX-2, offering insights into its underlying mechanisms. This study highlights the potential of M2pep-Cs-Cur NPs to reverse M2 polarization in TAMs, providing a promising targeted therapeutic strategy to overcome immunotherapy resistance and improve TNBC outcomes.
© 2025. The Author(s).
-
Cancer Research
-
Immunology and Microbiology
Local Exosome Inhibition Potentiates Mild Photothermal Immunotherapy Against Breast Cancer.
In Advanced Science (Weinheim, Baden-Wurttemberg, Germany) on 1 January 2025 by Chen, Q., Li, Y., et al.
Limited immune infiltration within the tumor microenvironment (TME) hampers the efficacy of immune checkpoint blockade (ICB) therapy. To enhance immune infiltration, mild photothermal therapy (PTT) is often combined with immunotherapy. However, the impact of mild PTT on the TME remains unclear. The bioinformatics analyses reveal that mild PTT amplifies immune cell infiltration and stimulates T-cell activity. Notably, it accelerates the release of tumor cell-derived exosomes (TEX) and upregulates PD-L1 expression on both tumor cells and TEX. Consequently, it is proposed that locally inhibiting TEX release is crucial for overcoming the adverse effects of mild PTT, thereby enhancing ICB therapy. Thus, a multi-stage drug delivery system is designed that concurrently delivers photosensitizers (reduced graphene oxide nanosheets, NRGO), anti-PD-L1 antibodies, and exosome inhibitors (sulfisoxazole). The system employs a temperature-sensitive lipid gel as the primary carrier, with NRGO serving as a secondary carrier that supports photothermal conversion and incorporation of sulfisoxazole. Importantly, controlled drug release is achieved using near-infrared radiation. The findings indicate that this local combination therapy remodels the immunosuppressive TME through exosome inhibition and enhanced immune cell infiltration, while also boosting T-cell activity to trigger systemic antitumor immunity, showcasing the remarkable efficacy of this combination strategy in eradicating cold tumors.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
-
Cancer Research
-
Immunology and Microbiology
Cell shape sensing licenses dendritic cells for homeostatic migration to lymph nodes.
In Nature Immunology on 1 July 2024 by Alraies, Z., Rivera, C. A., et al.
Immune cells experience large cell shape changes during environmental patrolling because of the physical constraints that they encounter while migrating through tissues. These cells can adapt to such deformation events using dedicated shape-sensing pathways. However, how shape sensing affects immune cell function is mostly unknown. Here, we identify a shape-sensing mechanism that increases the expression of the chemokine receptor CCR7 and guides dendritic cell migration from peripheral tissues to lymph nodes at steady state. This mechanism relies on the lipid metabolism enzyme cPLA2, requires nuclear envelope tensioning and is finely tuned by the ARP2/3 actin nucleation complex. We also show that this shape-sensing axis reprograms dendritic cell transcription by activating an IKKβ-NF-κB-dependent pathway known to control their tolerogenic potential. These results indicate that cell shape changes experienced by immune cells can define their migratory behavior and immunoregulatory properties and reveal a contribution of the physical properties of tissues to adaptive immunity.
© 2024. The Author(s).
-
Immunology and Microbiology
Cell Shape Sensing Licenses Dendritic Cells for Homeostatic Migration to Lymph Nodes
Preprint on Research Square on 11 April 2024 by Alraies, Z.
Immune cells experience large deformation events while patrolling their environment. These cell shape changes arise from the physical constraints encountered during migration within and between tissues. It has become increasingly clear that these cells can survive and adapt to these changes in cell shape using dedicated shape sensing pathways. However, how shape sensing impacts their behavior and function remains largely unknown. Here we identify a shape sensing mechanism that couples dendritic cell motility to expression of CCR7, the chemokine receptor guiding their migration from the periphery to lymph nodes. We found that this mechanism relies on the lipid metabolism enzyme cPLA 2 , requires nuclear envelop unfolding and tension and is finely tuned by the Arp2/3 actin nucleation complex. We further observed that shape sensing through the Arp2/3-cPLA 2 axis leads to Ikkb-NFkB-dependent transcriptional reprogramming of dendritic cells, which was shown to control their migration to lymph node and tolerogenic function at steady-state. Our results highlight that cell shape changes experienced by immune cells can define their migratory behavior and immunoregulatory properties, revealing the contribution of the physical properties of tissues to adaptive immunity.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
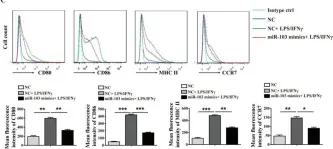
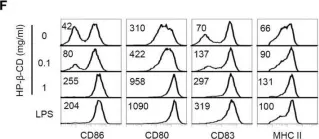
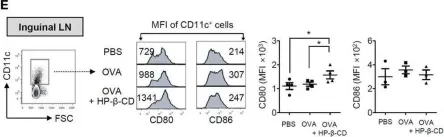
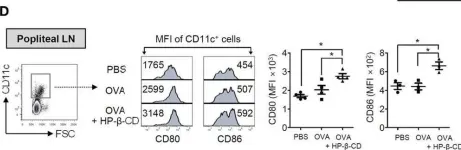
In Int J Biol Sci on 19 June 2020 by Zhu, X., Liu, H., et al.
Fig.4.C

-
FC/FACS
-
Collected and cropped from Int J Biol Sci by CiteAb, provided under a CC-BY license
Image 1 of 7
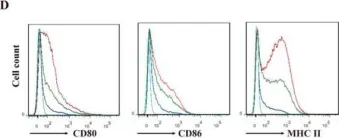
In Int J Biol Sci on 19 June 2020 by Zhu, X., Liu, H., et al.
Fig.4.D

-
FC/FACS
-
Collected and cropped from Int J Biol Sci by CiteAb, provided under a CC-BY license
Image 1 of 7
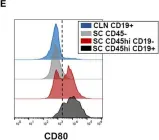
In Front Immunol on 21 August 2019 by DiSano, K. D., Royce, D. B., et al.
Fig.5.E

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 7
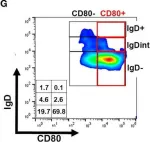
In Front Immunol on 21 August 2019 by DiSano, K. D., Royce, D. B., et al.
Fig.5.G

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 7
In Front Immunol on 5 November 2016 by Kim, S. K., Yun, C. H., et al.
Fig.5.F

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 7
In Front Immunol on 5 November 2016 by Kim, S. K., Yun, C. H., et al.
Fig.5.E

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 7
In Front Immunol on 5 November 2016 by Kim, S. K., Yun, C. H., et al.
Fig.5.D

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 7