B cells contribute to innate and adaptive immunity. In the former, Toll-like receptor (TLR) activation promotes the expansion of inflammatory B cells. In the latter, B cell receptor (BCR) activation results in the production of antibodies or autoantibodies. Antigen processing and presentation are closely associated with major histocompatibility class II (MHC-II) and its companion protein, class II invariant peptide (CLIP). The impact of autophagy on the regulation of these unique mechanisms of B cell activation and subset expansion has not been fully explored. The results from the current study show that activating autophagy with rapamycin (RAPA) or inhibiting autophagy with hydroxycholoroquine (HCQ) differentially influences the TLR9 and BCR activation of B cells. These differences include the selective expansion of B1 and B2 B cell subsets, the regulation of the cell-surface expression of MHC-II and CLIP, and the ability of distinct B cell subsets to present peptide antigens. These novel findings demonstrate that the unique B cell activation mechanisms induced by TLR9 and BCR activation are differentially influenced by RAPA and HCQ, owing to the selective modulation of B cell subset expansion, and antigen processing and presentation by MHC-II proteins.
Product Citations: 220
In International Journal of Molecular Sciences on 24 June 2025 by Peddaboina, C., Iannucci, J., et al.
-
Cell Biology
-
Immunology and Microbiology
In IScience on 19 May 2023 by Song, J., Deshpande, T., et al.
Germinal center (GC) formation and antibody production in lymph node follicles require coordinated interactions between B-cells, T-cells and dendritic cells (DCs), orchestrated by the extracellular matrix-rich reticular fiber (RF) network. We describe a unique laminin 523-containing RF network around and between follicles that associates with PDGFrecβhighCCL19lowgp38low fibroblastic reticular cells (FRC). In the absence of FRC expression of laminin α5 (pdgfrb-cre:Lama5fl/fl), pre-Tfh-cells, B-cells and DCs are displaced from follicle borders, correlating with fewer Tfh-cells and GC B-cells. Total DCs are not altered in pdgfrb-cre:Lama5fl/fl mice, but cDC2s, which localize to laminin α5 in RFs at follicle borders, are reduced. In addition, PDGFrecβhighCCL19lowgp38low FRCs show lower Ch25h expression, required for 7α,25-dihydroxycholesterol synthesis that attracts pre-Tfh-cells, B-cells and DCs to follicle borders. We propose that RF basement membrane components represent a type of tissue memory that guides the localization and differentiation of both specialized FRC and DC populations, required for normal lymph node function.
© 2023 The Author(s).
-
Mus musculus (House mouse)
In Nature Communications on 29 March 2022 by Yang, N., Luna, J. M., et al.
The pulmonary immune system consists of a network of tissue-resident cells as well as immune cells that are recruited to the lungs during infection and/or inflammation. How these immune components function during an acute poxvirus infection is not well understood. Intranasal infection of mice with vaccinia virus causes lethal pneumonia and systemic dissemination. Here we report that vaccinia C7 is a crucial virulence factor that blocks activation of the transcription factor IRF3. We provide evidence that type II alveolar epithelial cells (AECIIs) respond to pulmonary infection of vaccinia virus by inducing IFN-β and IFN-stimulated genes via the activation of the MDA5 and STING-mediated nucleic acid-sensing pathways and the type I IFN positive feedback loop. This leads to the recruitment and activation of CCR2+ inflammatory monocytes in the infected lungs and subsequent differentiation into Lyve1- interstitial macrophages (Lyve1- IMs), which efficiently engulf viral particles and block viral replication. Our results provide insights into how innate immune sensing of viral infection by lung AECIIs influences the activation and differentiation of CCR2+ inflammatory monocytes to defend against pulmonary poxvirus infection.
© 2022. The Author(s).
-
Immunology and Microbiology
T cell migration requires ion and water influx to regulate actin polymerization
Preprint on BioRxiv : the Preprint Server for Biology on 18 March 2022 by de Boer, L. L., Melgrati, S., et al.
Migration of T cells is essential for their ability to mount immune responses. Chemokine-induced T cell migration requires WNK1, a kinase that regulates ion influx into the cell. However, it is not known why ion entry is necessary for T cell movement. Here we show that signaling from the chemokine receptor CCR7 leads to activation of WNK1 and its downstream pathway at the leading edge of migrating CD4 + T cells, resulting, we propose, in ion influx and consequent water entry by osmosis through AQP3. This WNK1-induced water entry is required to swell the membrane at the leading edge, generating space into which actin filaments can polymerize, thereby facilitating forward movement of the cell. Given the broad expression of WNK1 pathway proteins, our study shows that ion and water influx are likely to be essential for migration in many cell types, including leukocytes and metastatic tumor cells. h4>One Sentence Summary/h4> Chemokine-induced migration of T cells requires water entry at the leading edge to facilitate actin polymerization.
-
Cell Biology
-
Immunology and Microbiology
Differential location of NKT and MAIT cells within lymphoid tissue.
In Scientific Reports on 8 March 2022 by Johnson, D. N., Ruan, Z., et al.
Natural Killer T (NKT) cells and Mucosal-Associated Invariant T (MAIT) cells are innate-like T cells that express semi-invariant αβ T cell receptors (TCRs) through which they recognise CD1d and MR1 molecules, respectively, in complex with specific ligands. These cells play important roles in health and disease in many organs, but their precise intra-organ location is not well established. Here, using CD1d and MR1 tetramer staining techniques, we describe the precise location of NKT and MAIT cells in lymphoid and peripheral organs. Within the thymus, NKT cells were concentrated in the medullary side of the corticomedullary junction. In spleen and lymph nodes, NKT cells were mainly localised within T cell zones, although following in vivo activation with the potent NKT-cell ligand α-GalCer, they expanded throughout the spleen. MAIT cells were clearly detectable in Vα19 TCR transgenic mice and were rare but detectable in lymphoid tissue of non-transgenic mice. In contrast to NKT cells, MAIT cells were more closely associated with the B cell zone and red pulp of the spleen. Accordingly, we have provided an extensive analysis of the in situ localisation of NKT and MAIT cells and suggest differences between the intra-organ location of these two cell types.
© 2022. The Author(s).
In Cells on 31 January 2022 by Barington, L., Christensen, L. V. V., et al.
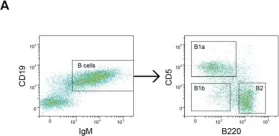
Fig.1.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 5
In Front Immunol on 22 August 2018 by Hobeika, E., Dautzenberg, M., et al.
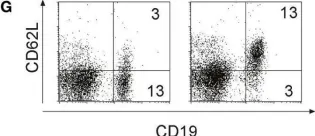
Fig.4.G

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 5
In Front Immunol on 22 August 2018 by Hobeika, E., Dautzenberg, M., et al.
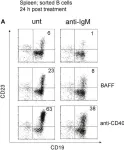
Fig.6.A

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 5
In PLoS One on 25 April 2013 by Torrado, E., Fountain, J. J., et al.
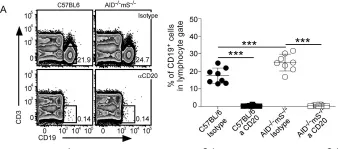
Fig.2.A

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 5
In PLoS Pathog on 1 October 2009 by Bai, F., Town, T., et al.
Fig.8.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS Pathog by CiteAb, provided under a CC-BY license
Image 1 of 5