Respiratory syncytial virus (RSV) ranks as the second leading cause of infant death globally and a significant contributor to morbidity and mortality among adults over 60 years old. The development of effective RSV vaccines and immunoprophylaxis remains a key focus. In our research, we formulated a protein-based vaccine known as MF59/preF, which combines the RSV pre-fusion (preF) antigen with an MF59-like oil-in-water adjuvant. Intramuscular (IM) or intranasal (IN) immunization of the MF59-adjuvanted preF protein vaccine elicited robust immune responses and neutralizing antibodies against both RSV A2 and RSV B strains, with the IM showing a particularly pronounced effect. Notably, IN immunization with MF59/preF demonstrated superior mucosal immunity, characterized by elevated levels of IgA antibodies and an increased frequency of tissue-resident memory T (TRM) cells locally. More importantly, the combined IM and IN delivery of the MF59/preF vaccine synergistically enhanced antigen-specific humoral and cellular immune responses at both systemic and mucosal sites. Our study highlights the crucial impact of the route of administration and adjuvanted-protein subunit vaccines on triggering strong humoral and cellular immunity in mice.
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Product Citations: 123
In MedComm (2020) on 1 August 2025 by Shi, J., Lei, H., et al.
-
Immunology and Microbiology
In IScience on 18 April 2025 by Pioli, K. T., Ghosh, S., et al.
Mutations that negatively impact mitochondrial function are highly prevalent in humans and lead to disorders with a wide spectrum of disease phenotypes, including deficiencies in immune cell development and/or function. Previous analyses of mice with a hepatocyte-specific cytochrome c oxidase (COX) deficiency revealed an unexpected peripheral blood leukopenia associated with splenic and thymic atrophy. Here, we use mice with a hepatocyte-specific deletion of the COX assembly factor Sco1 to show that metabolic defects extrinsic to the hematopoietic compartment lead to a pan-lymphopenia represented by severe losses in both B and T cells. We further demonstrate that immune defects in these mice are associated with the loss of bone marrow lymphoid progenitors common to both lineages and early signs of autoantibody-mediated autoimmunity. Our findings collectively identify hepatocyte dysfunction as a potential instigator of immunodeficiency in patients with congenital mitochondrial defects who suffer from chronic or recurrent infections.
© 2025 The Author(s).
In Nature Communications on 5 February 2025 by Svedlund Eriksson, E., Lantero Rodriguez, M., et al.
Men develop larger infarct sizes than women after a myocardial infarction (MI), but the mechanism underlying this sex difference is unknown. Here, we demonstrated that blood neutrophil counts post-MI were higher in male than female mice. Castration-induced testosterone deficiency reduced blood neutrophil counts to the level in females and increased survival post-MI. These effects were mimicked by Osterix-directed ablation of the androgen receptor in bone marrow (BM). Mechanistically, androgens downregulated the leukocyte retention factor CXCL12 in BM stromal cells. Post-hoc analysis of clinical trial data showed that neutrophilia was greater in men than women after reperfusion of first-time ST-elevation MI, and tocilizumab, an interleukin-6 receptor inhibitor, reduced blood neutrophil counts and infarct size to a greater extent in men than women. Our work reveals a previously unknown mechanism connecting testosterone with neutrophilia and MI injury via BM and identifies the importance of considering sex when developing anti-inflammatory strategies to treat MI.
© 2025. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Cardiovascular biology
-
Endocrinology and Physiology
In MedComm (2020) on 1 September 2024 by Peng, D., He, C., et al.
The newly identified XBB.1.16-containing sublineages, including XBB.1.5, have become the prevailing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant in circulation. Unlike previous Omicron XBB variants (e.g., XBB.1.5 and XBB.1.9) harboring the F486P substitution, XBB.1.16 also carries a T478R substitution in the receptor-binding domain (RBD). Numerous researchers have delved into the high transmissibility and immune evasion of XBB.1.16 subvariant. Therefore, developing a new vaccine targeting XBB.1.16, including variants of concern (VOCs), is paramount. In our study, we engineered a recombinant protein by directly linking the S-RBD sequence of the XBB.1.16 strain of SARS-CoV-2 to the sequences of two heptad repeat sequences (HR1 and HR2) from the SARS-CoV-2 S2 subunit. Named the recombinant RBDXBB.1.16-HR/trimeric protein, this fusion protein autonomously assembles into a trimer. Combined with an MF59-like adjuvant, the RBDXBB.1.16-HR vaccine induces a robust humoral immune response characterized by high titers of neutralizing antibodies against variant pseudovirus and authentic VOCs and cellular immune responses. Additionally, a fourth heterologous RBDXBB.1.16-HR vaccine enhances both humoral and cellular immune response elicited by three-dose mRNA vaccines. These findings demonstrate that the recombinant RBDXBB.1.16-HR protein, featuring the new T478R mutation, effectively induces solid neutralizing antibodies to combat newly emerged XBB variants.
© 2024 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Vaccines on 18 July 2024 by Eom, G. D., Chu, K. B., et al.
Cutaneous leishmaniasis (CL) is a tropical disease endemic in many parts of the world. Characteristic clinical manifestations of CL include the formation of ulcerative skin lesions that can inflict life-long disability if left untreated. Although drugs are available, they are unaffordable and out of reach for individuals who need them the most. Developing a highly cost-efficient CL vaccine could address this problem but such a vaccine remains unavailable. Here, we developed a chimeric influenza virus-like particle expressing the Leishmania amazonensis promastigote surface antigen (LaPSA-VLP). LaPSA-VLPs were self-assembled in Spodoptera frugiperda insect cell lines using the baculovirus expression system. After characterizing the vaccines and confirming successful VLP assembly, BALB/c mice were immunized with these vaccines for efficacy assessment. Sera acquired from mice upon subcutaneous immunization with the LaPSA-VLP specifically interacted with the L. amazonensis soluble total antigens. LaPSA-VLP-immunized mice elicited significantly greater quantities of parasite-specific IgG from the spleens, popliteal lymph nodes, and footpads than unimmunized mice. LaPSA-VLP immunization also enhanced the proliferation of B cell populations in the spleens of mice and significantly lessened the CL symptoms, notably the footpad swelling and IFN-γ-mediated inflammatory response. Overall, immunizing mice with the LaPSA-VLPs prevented mice from developing severe CL symptoms, signifying their developmental potential.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In PLoS One on 2 February 2016 by Kicielińska, J., Szczygieł, A., et al.
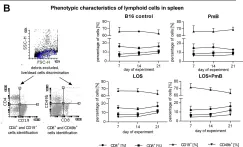
Fig.6.B

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2
In Stem Cell Reports on 10 November 2015 by Ikawa, T., Masuda, K., et al.
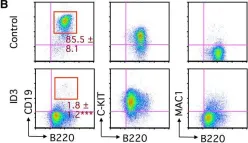
Fig.1.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Stem Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 2