Chimeric antigen receptor T (CAR-T) cells show remarkable efficacy for some hematological malignancies. However, CAR targets that are expressed at high level and selective to tumors are scarce. Several strategies have been proposed to tackle the on-target off-tumor toxicity of CAR-T cells that arise from suboptimal selectivity, but these are complicated, with many involving dual gene expression for specificity. In this study, we show that switchable CAR-T cells with a tumor targeting adaptor can mitigate on-target off-tumor toxicity against a low selectivity tumor antigen that cannot be targeted by conventional CAR-T cells, such as CD40. Our system is composed of anti-cotinine murine CAR-T cells and cotinine-labeled anti-CD40 single chain variable fragments (scFv), with which we show selective tumor killing while sparing CD40-expressing normal cells including macrophages in a mouse model of lymphoma. Simple replacement of the tumor-targeting adaptor with a suicidal drug-conjugated tag may further enhance safety by enabling permanent in vivo depletion of the switchable CAR-T cells when necessary. In summary, our switchable CAR system can control CAR-T cell toxicity while maintaining therapeutic efficacy, thereby expanding the range of CAR targets.
© 2024. The Author(s).
Product Citations: 75
In Nature Communications on 18 November 2024 by Park, H. B., Kim, K. H., et al.
-
Immunology and Microbiology
In Aging Cell on 1 June 2024 by Fisher, J. S., Adán-Barrientos, I., et al.
Weakened germinal center responses by the aged immune system result in diminished immunity against pathogens and reduced efficacy of vaccines. Prolonged contacts between activated B cells and CD4+ T cells are crucial to germinal center formation and T follicular helper cell (Tfh) differentiation, but it is unclear how aging impacts the quality of this interaction. Peptide immunization confirmed that aged mice have decreased expansion of antigen-specific germinal center B cells and reduced antibody titers. Furthermore, aging was associated with accumulated Tfh cells, even in naïve mice. Despite increased numbers, aged Tfh had reduced expression of master transcription factor BCL6 and increased expression of the ectonucleotidase CD39. In vitro activation revealed that proliferative capacity was maintained in aged CD4+ T cells, but not the costimulatory molecule CD40L. When activated in vitro by aged antigen-presenting cells, young CD4+ naïve T cells generated reduced numbers of activated cells with upregulated CD40L. To determine the contribution of cell-extrinsic influences on antigen-specific Tfh induction, young, antigen-specific B and CD4+ T cells were adoptively transferred into aged hosts prior to peptide immunization. Transferred cells had reduced expansion and differentiation into germinal center B cell and Tfh and reduced antigen-specific antibody titers when compared to young hosts. Young CD4+ T cells transferred aged hosts differentiated into Tfh cells with reduced PD-1 and BCL6 expression, and increased CD39 expression, though they maintained their mitochondrial capacity. These results highlight the role of the lymphoid microenvironment in modulating CD4+ T cell differentiation, which contributes to impaired establishment and maintenance of germinal centers.
© 2024 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
-
Mus musculus (House mouse)
-
Cell Biology
-
Immunology and Microbiology
Blocking CD40 Alleviates Th1 and Th17 Cell Responses in Elastin Peptide-Induced Murine Emphysema.
In International Journal of Chronic Obstructive Pulmonary Disease on 29 November 2023 by Ma, T., Zhang, H., et al.
To investigate the role of the CD40-CD40 ligand (CD40L) pathway in the regulation of Th1, Th17, and regulatory T (Treg)-cell responses in an elastin peptide (EP)-induced autoimmune emphysema mouse model.
BALB/c mice were transnasally treated with EP on day 0, injected intravenously with anti-CD40 antibody via the tail vein on day 33, and sacrificed on day 40. The severity of emphysema was evaluated by determining the mean linear intercept (MLI) and destructive index (DI) from lung sections. The proportions of myeloid dendritic cells (mDCs) and Th1, Th17, and Treg cells in the blood, spleen, and lungs were determined via flow cytometry. The levels of the cytokines interleukin (IL)-6, IL-17, interferon (IFN)-γ, and transforming growth factor (TGF)-β were detected via enzyme-linked immunosorbent assay. Ifnγ, IL17a, Rorγt and Foxp3 transcription levels were detected via polymerase chain reaction.
CD40+ mDCs accumulated in the lungs of EP-stimulated mice. Blocking the CD40-CD40L pathway with an anti-CD40 antibody alleviated Th1 and Th17 responses; increased the proportion of Treg cells; decreased MLI and DI; reduced the levels of cytokines IL-6, IL-17, and IFN-γ as well as the transcription levels of Ifnγ, IL17a, and Rorγt; and upregulated the expression of TGF-β and Foxp3.
The CD40-CD40L pathway could play a critical role in Th1, Th17 and Treg cell dysregulation in EP-mediated emphysema and could be a potential therapeutic target.
© 2023 Ma et al.
-
FC/FACS
-
Mus musculus (House mouse)
In Acta Pharmaceutica Sinica. B on 1 September 2023 by Zhao, H., Li, Y., et al.
Activating humoral and cellular immunity in lymph nodes (LNs) of nanoparticle-based vaccines is critical to controlling tumors. However, how the physical properties of nanovaccine carriers orchestrate antigen capture, lymphatic delivery, antigen presentation and immune response in LNs is largely unclear. Here, we manufactured gold nanoparticles (AuNPs) with the same size but different shapes (cages, rods, and stars), and loaded tumor antigen as nanovaccines to explore their disparate characters on above four areas. Results revealed that star-shaped AuNPs captured and retained more repetitive antigen epitopes. On lymphatic delivery, both rods and star-shaped nanovaccines mainly drain into the LN follicles region while cage-shaped showed stronger paracortex retention. A surprising finding is that the star-shaped nanovaccines elicited potent humoral immunity, which is mediated by CD4+ T helper cell and follicle B cell cooperation significantly preventing tumor growth in the prophylactic study. Interestingly, cage-shaped nanovaccines preferentially presented peptide-MHC I complexes to evoke robust CD8+ T cell immunity and showed the strongest therapeutic efficacy when combined with the PD-1 checkpoint inhibitor in established tumor study. These results highlight the importance of nanoparticle shape on antigen delivery and presentation for immune response in LNs, and our findings support the notion that different design strategies are required for prophylactic and therapeutic vaccines.
© 2023 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Journal for Immunotherapy of Cancer on 1 August 2023 by Alshebremi, M., Tomchuck, S. L., et al.
Despite its potential utility in delivering direct tumor killing and in situ whole-cell tumor vaccination, tumor cryoablation produces highly variable and unpredictable clinical response, limiting its clinical utility. The mechanism(s) driving cryoablation-induced local antitumor immunity and the associated abscopal effect is not well understood.
The aim of this study was to identify and explore a mechanism of action by which cryoablation enhances the therapeutic efficacy in metastatic tumor models. We used the subcutaneous mouse model of the rhabdomyosarcoma (RMS) cell lines RMS 76-9STINGwt or RMS 76-9STING-/-, along with other murine tumor models, in C57BL/6 or STING-/- (TMEM173-/- ) mice to evaluate local tumor changes, lung metastasis, abscopal effect on distant tumors, and immune cell dynamics in the tumor microenvironment (TME).
The results show that cryoablation efficacy is dependent on both adaptive immunity and the STING signaling pathway. Contrary to current literature dictating an essential role of host-derived STING activation as a driver of antitumor immunity in vivo, we show that local tumor control, lung metastasis, and the abscopal effect on distant tumor are all critically dependent on a functioning tumor cell-intrinsic STING signaling pathway, which induces inflammatory chemokine and cytokine responses in the cryoablated TME. This reliance extends beyond cryoablation to include intratumoral STING agonist therapy. Additionally, surveys of gene expression databases and tissue microarrays of clinical tumor samples revealed a wide spectrum of expressions among STING-related signaling components.
Tumor cell-intrinsic STING pathway is a critical component underlying the effectiveness of cryoablation and suggests that expression of STING-related signaling components may serve as a potential therapy response biomarker. Our data also highlight an urgent need to further characterize tumor cell-intrinsic STING pathways and the associated downstream inflammatory response evoked by cryoablation and other STING-dependent therapy approaches.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
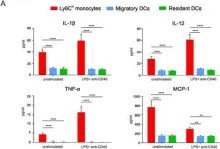
In Sci Rep on 20 July 2017 by De Koker, S., Van Hoecke, L., et al.
Fig.7.A

-
FC/FACS
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 2
In PLoS Negl Trop Dis on 10 January 2013 by Choi, J. H., Cheong, T. C., et al.
Fig.1.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS Negl Trop Dis by CiteAb, provided under a CC-BY license
Image 1 of 2