Macrophages are crucial in the body's inflammatory response, with tightly regulated functions for optimal immune system performance. Our study reveals that the RAS-p110α signalling pathway, known for its involvement in various biological processes and tumourigenesis, regulates two vital aspects of the inflammatory response in macrophages: the initial monocyte movement and later-stage lysosomal function. Disrupting this pathway, either in a mouse model or through drug intervention, hampers the inflammatory response, leading to delayed resolution and the development of more severe acute inflammatory reactions in live models. This discovery uncovers a previously unknown role of the p110α isoform in immune regulation within macrophages, offering insight into the complex mechanisms governing their function during inflammation and opening new avenues for modulating inflammatory responses.
© 2024, Rosell, Krygowska et al.
Product Citations: 215
In eLife on 24 April 2025 by Rosell, A., Krygowska, A. A., et al.
-
ICC-IF
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Mitochondrial complexity is regulated at ER-mitochondria contact sites via PDZD8-FKBP8 tethering.
In Nature Communications on 17 April 2025 by Nakamura, K., Aoyama-Ishiwatari, S., et al.
Mitochondria-ER membrane contact sites (MERCS) represent a fundamental ultrastructural feature underlying unique biochemistry and physiology in eukaryotic cells. The ER protein PDZD8 is required for the formation of MERCS in many cell types, however, its tethering partner on the outer mitochondrial membrane (OMM) is currently unknown. Here we identify the OMM protein FKBP8 as the tethering partner of PDZD8 using a combination of unbiased proximity proteomics, CRISPR-Cas9 endogenous protein tagging, Cryo-electron tomography, and correlative light-electron microscopy. Single molecule tracking reveals highly dynamic diffusion properties of PDZD8 along the ER membrane with significant pauses and captures at MERCS. Overexpression of FKBP8 is sufficient to narrow the ER-OMM distance, whereas independent versus combined deletions of these two proteins demonstrate their interdependence for MERCS formation. Furthermore, PDZD8 enhances mitochondrial complexity in a FKBP8-dependent manner. Our results identify a novel ER-mitochondria tethering complex that regulates mitochondrial morphology in mammalian cells.
© 2025. The Author(s).
-
Cell Biology
Contribution of Sorting Nexin 3 in the Cytomegalovirus Assembly.
In Biomedicines on 11 April 2025 by Viduka, I., Stimac, I., et al.
Background/Objectives: Cytomegalovirus (CMV) infection expands early endosomes (EEs) into tubular extensions that may contribute to the control of virus replication and virion assembly. Sequential recruitment of protein coats and sorting nexins (SNXs) creates membrane zones at the EEs that serve as scaffolds for membrane tubulation and retrieval of cargo proteins, including host cell signaling proteins and viral glycoproteins. This study aims to investigate whether the SNX3-dependent zone of EEs contributes to CMV replication and assembly. Methods: Protein localization was analyzed by confocal imaging and expression by Western blot. The contribution of SNX3 to murine CMV (MCMV) replication, assembly compartment (AC) formation, and virion release was analyzed by siRNA and shRNA depletion. The impact of other downstream SNXs that act in EE tubulation was investigated by combined siRNA knockdowns of SNX1, SNX2, SNX4, SNX17, and SNX27 on cell lines expressing shRNA for SNX3. Results: The SNX3-162 isoform acting at EEs was efficiently knocked down by siRNA and shRNA. The SNX3-dependent EE zone recruited SNX27 and contributed to Rab10-dependent tubulation within the pre-AC. SNX3 was not essential for MCMV replication but contributed to the SNX27-, SNX17- and SNX4-dependent release of virions. Silencing SNX3 further reduced the release of virions after silencing SNX27, SNX4, and SNX17, three SNXs that control recycling to the plasma membrane. Conclusions: SNX3 contributes to the formation of pre-AC and MCMV assembly. It acts sequentially with SNX27, SNX4, and SNX17 along the recycling pathway in the process of the production and release of infection virions, suggesting that multiple membrane sources may contribute to the secondary envelopment of MCMV virions.
-
Immunology and Microbiology
In JCI Insight on 24 March 2025 by Waich, S., Kreidl, K., et al.
The osteo-oto-hepato-enteric (O2HE) syndrome is a severe autosomal recessive disease ascribed to loss-of-function mutations in the Unc-45 myosin chaperone A (UNC45A) gene. The clinical spectrum includes bone fragility, hearing loss, cholestasis, and life-threatening diarrhea associated with microvillus inclusion disease-like enteropathy. Here, we present molecular and functional analysis of the UNC45A c.710T>C (p.Leu237Pro) missense variant, which revealed a unique pathogenicity compared with other genetic variants causing UNC45A deficiency. The UNC45A p.Leu237Pro mutant retained chaperone activity, prevented myosin aggregation, and supported proper nonmuscle myosin II (NMII) filament formation in patient fibroblasts and human osteosarcoma (U2OS) cells. However, the mutant formed atypically stable oligomers and prevented chaperone-myosin complex dissociation, thereby inhibiting NMII functions. Similar to biallelic UNC45A deficiency, this resulted in impaired intracellular trafficking, defective recycling, and abnormal retention of transferrin at various endocytic sites. In particular, coexpression of wild-type protein attenuated the pathogenic effects of the variant by inhibiting excessive oligomer formation. Our results elucidate the pathogenic mechanisms and recessive characteristics of this variant and may aid in the development of targeted therapies.
-
WB
-
Homo sapiens (Human)
Preprint on Research Square on 20 March 2025 by Wang, Y., Moura, A. K., et al.
Abstract Niemann-Pick Disease (NPD) is a rare autosomal recessive lysosomal storage disorder (LSD) caused by the deficiency of acid sphingomyelinase (ASMD), which is encoded by the Smpd1 gene. ASMD impacts multiple organ systems in the body, including the cardiovascular system. This study is the first to characterize cardiac pathological changes in ASMD mice under baseline conditions, offering novel insights into the cardiac implications of NPD. Using histological analysis, biochemical assays, and echocardiography, we assessed cardiac pathological changes and function in Smpd1−/− mice compared to Smpd1+/+ littermate controls. Immunofluorescence and biochemical assays demonstrated that ASMD induced lysosomal dysfunction, as evidenced by the accumulation of lysosomal-associated membrane proteins, lysosomal protease, and autophagosomes in pericytes and cardiomyocytes. This lysosomal dysfunction was accompanied by pericytes and cardiomyocytes inflammation, characterized by increased expression of caspase1 and inflammatory cytokines, and infiltration of inflammatory cells in the cardiac tissues of Smpd1−/− mice. In addition, histological analysis revealed increased lipid deposition and cardiac steatosis, along with pericyte-to-myofibroblast transition (PMT) and interstitial fibrosis in Smpd1−/− mice. Moreover, echocardiography further demonstrated that Smpd1−/− mice developed coronary microvascular dysfunction (CMD), as evidenced by decreased coronary blood flow velocity and increased coronary arteriolar wall thickness. Additionally, these mice exhibited significant impairments in systolic and diastolic cardiac function, as shown by a reduced ejection fraction and prolonged left ventricular relaxation time constant (Tau value). These findings suggest that ASMD induces profound pathological changes and vascular dysfunction in the myocardium, potentially driven by mechanisms involving lysosomal dysfunction as well as both pericytes and cardiac inflammation.
-
Cardiovascular biology
-
Cell Biology
In JCI Insight on 22 August 2023 by Meena, N. K., Randazzo, D., et al.
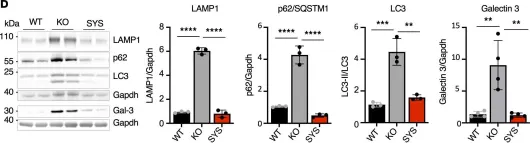
Fig.2.D

-
WB
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 13
In JCI Insight on 22 August 2023 by Meena, N. K., Randazzo, D., et al.
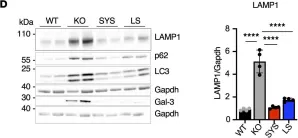
Fig.5.D

-
WB
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 13
In JCI Insight on 22 August 2023 by Meena, N. K., Randazzo, D., et al.
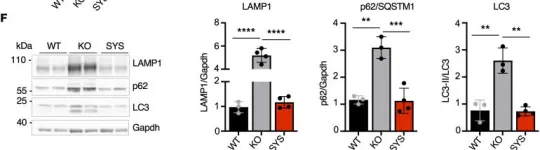
Fig.9.F

-
WB
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 13
In Exp Mol Med on 1 July 2023 by Rah, S. Y., Joe, Y., et al.
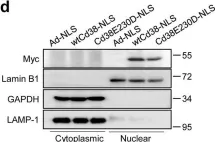
Fig.1.D

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Exp Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 13
In Exp Mol Med on 1 July 2023 by Rah, S. Y., Joe, Y., et al.
Fig.2.E

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Exp Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 13
In Sci Adv on 5 May 2023 by Zhang, T., Pang, W., et al.
Fig.4.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Sci Adv by CiteAb, provided under a CC-BY license
Image 1 of 13
In Front Microbiol on 14 February 2023 by Matsumura, K., Takaki, S., et al.
Fig.4.C

-
ICC-IF
-
Collected and cropped from Front Microbiol by CiteAb, provided under a CC-BY license
Image 1 of 13
In Acta Neuropathol Commun on 14 March 2022 by Feng, T., Luan, L., et al.
Fig.4.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Acta Neuropathol Commun by CiteAb, provided under a CC-BY license
Image 1 of 13
In Acta Neuropathol Commun on 1 April 2021 by Frew, J. & Nygaard, H. B.
Fig.3.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Acta Neuropathol Commun by CiteAb, provided under a CC-BY license
Image 1 of 13
In Int J Mol Sci on 8 December 2020 by Tran, M. T., Okusha, Y., et al.
Fig.6.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 13
In EMBO Rep on 5 October 2020 by Feng, T., Mai, S., et al.
Fig.8.G

-
WB
-
Mus musculus (House mouse)
Collected and cropped from EMBO Rep by CiteAb, provided under a CC-BY license
Image 1 of 13
In Elife on 4 May 2017 by Kleinecke, S., Richert, S., et al.
Fig.3.F

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 13
In Nat Commun on 11 March 2016 by Bartuzi, P., Billadeau, D. D., et al.
Fig.5.A

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 13