Missense variants can have pleiotropic effects on protein function and predicting these effects can be difficult. We performed near-saturation deep mutational scanning of P2RY8, a G-protein-coupled receptor that promotes germinal center B cell confinement. We assayed the effect of each variant on surface expression, migration, and proliferation. We delineated variants that affected both expression and function, affected function independently of expression, and discrepantly affected migration and proliferation. We also used cryo-electron microscopy to determine the structure of activated, ligand-bound P2RY8, providing structural insights into the effects of variants on ligand binding and signal transmission. We applied the deep mutational scanning results to both improve computational variant effect predictions and to characterize the phenotype of germline variants and lymphoma-associated variants. Together, our results demonstrate the power of integrating deep mutational scanning, structure determination, and in silico prediction to advance the understanding of a receptor important in human health.
Product Citations: 31
Phenotypic pleiotropy of missense variants in human B cell-confinement receptor P2RY8
Preprint on BioRxiv : the Preprint Server for Biology on 6 March 2025 by LaFlam, T. N., Billesbølle, C. B., et al.
-
Immunology and Microbiology
In IScience on 18 October 2024 by Fahlquist-Hagert, C., Wittenborn, T. R., et al.
Autoantibodies generated in germinal centers (GCs) contribute to the pathogenesis of autoimmune diseases. GCs are controlled by specialized FoxP3+ T-follicular regulatory cells (Tfr), but their role in established autoimmunity is unclear. We generated autoimmune bone marrow chimeras in which Tfr could be specifically ablated by diphtheria toxin. Furthermore, we tracked the clonal persistence and evolution of Tfr populations using Confetti reporters. Ablation of Tfr caused increased early plasma cell output, but longer-term ablation did not increase plasma cell levels beyond those of Tfr-sufficient controls, suggesting that Tfr fail to contain chronic autoreactive GC responses. In agreement, Tfr were robustly induced in early autoreactive GCs but then waned. Moreover, we observed polyclonal Tfr expansion when ablating part of the Tfr subset. Hence, under homeostatic conditions, a polyclonal population of Tfr operates to control autoreactivity by limiting the output of plasma cells from GCs, but in chronic autoimmunity, this mechanism fails.
© 2024 The Author(s).
Fate mapping and scRNA sequencing reveal origin and diversity of lymph node stromal precursors.
In Immunity on 12 April 2022 by Lenti, E., Genovese, L., et al.
Lymph node (LN) stromal cells play a crucial role in LN development and in supporting adaptive immune responses. However, their origin, differentiation pathways, and transcriptional programs are still elusive. Here, we used lineage-tracing approaches and single-cell transcriptome analyses to determine origin, transcriptional profile, and composition of LN stromal and endothelial progenitors. Our results showed that all major stromal cell subsets and a large proportion of blood endothelial cells originate from embryonic Hoxb6+ progenitors of the lateral plate mesoderm (LPM), whereas lymphatic endothelial cells arise from Pax3+ progenitors of the paraxial mesoderm (PXM). Single-cell RNA sequencing revealed the existence of different Cd34+ and Cxcl13+ stromal cell subsets and showed that embryonic LNs contain proliferating progenitors possibly representing the amplifying populations for terminally differentiated cells. Taken together, our work identifies the earliest embryonic sources of LN stromal and endothelial cells and demonstrates that stromal diversity begins already during LN development.
Copyright © 2022 Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
High-affinity, neutralizing antibodies to SARS-CoV-2 can be made without T follicular helper cells.
In Science Immunology on 4 February 2022 by Chen, J. S., Chow, R. D., et al.
T follicular helper (TFH) cells are the conventional drivers of protective, germinal center (GC)–based antiviral antibody responses. However, loss of TFH cells and GCs has been observed in patients with severe COVID-19. As T cell–B cell interactions and immunoglobulin class switching still occur in these patients, noncanonical pathways of antibody production may be operative during SARS-CoV-2 infection. We found that both TFH-dependent and -independent antibodies were induced against SARS-CoV-2 infection, SARS-CoV-2 vaccination, and influenza A virus infection. Although TFH-independent antibodies to SARS-CoV-2 had evidence of reduced somatic hypermutation, they were still high affinity, durable, and reactive against diverse spike-derived epitopes and were capable of neutralizing both homologous SARS-CoV-2 and the B.1.351 (beta) variant of concern. We found by epitope mapping and B cell receptor sequencing that TFH cells focused the B cell response, and therefore, in the absence of TFH cells, a more diverse clonal repertoire was maintained. These data support an alternative pathway for the induction of B cell responses during viral infection that enables effective, neutralizing antibody production to complement traditional GC-derived antibodies that might compensate for GCs damaged by viral inflammation.
-
IHC-IF
-
Mus musculus (House mouse)
-
COVID-19
P2RY8 variants in lupus patients uncover a role for the receptor in immunological tolerance.
In The Journal of Experimental Medicine on 3 January 2022 by He, Y., Gallman, A. E., et al.
B cell self-tolerance is maintained through multiple checkpoints, including restraints on intracellular signaling and cell trafficking. P2RY8 is a receptor with established roles in germinal center (GC) B cell migration inhibition and growth regulation. Somatic P2RY8 variants are common in GC-derived B cell lymphomas. Here, we identify germline novel or rare P2RY8 missense variants in lupus kindreds or the related antiphospholipid syndrome, including a "de novo" variant in a child with severe nephritis. All variants decreased protein expression, F-actin abundance, and GPCR-RhoA signaling, and those with stronger effects increased AKT and ERK activity and cell migration. Remarkably, P2RY8 was reduced in B cell subsets from some SLE patients lacking P2RY8 gene variants. Low P2RY8 correlated with lupus nephritis and increased age-associated B cells and plasma cells. By contrast, P2RY8 overexpression in cells and mice restrained plasma cell development and reinforced negative selection of DNA-reactive developing B cells. These findings uncover a role of P2RY8 in immunological tolerance and lupus pathogenesis.
© 2021 He et al.
-
IHC-IF
-
Immunology and Microbiology
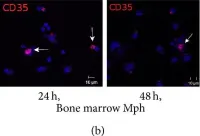
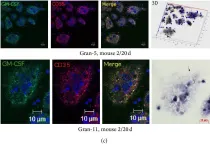
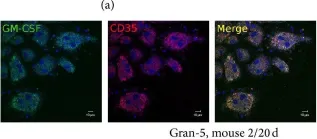
In J Immunol Res on 12 April 2016 by Ufimtseva, E.
Fig.10.B

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from J Immunol Res by CiteAb, provided under a CC-BY license
Image 1 of 3
In J Immunol Res on 12 April 2016 by Ufimtseva, E.
Fig.10.C

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from J Immunol Res by CiteAb, provided under a CC-BY license
Image 1 of 3
In J Immunol Res on 12 April 2016 by Ufimtseva, E.
Fig.10.A

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from J Immunol Res by CiteAb, provided under a CC-BY license
Image 1 of 3