Effector CD8+ T cells are important mediators of adaptive immunity, and receptor-ligand interactions that regulate their survival may have therapeutic potential. Here, we identified a subset of effector CD8+ T cells that expressed the inhibitory fragment crystallizable (Fc) receptor FcγRIIB following activation and multiple rounds of division. CD8+ T cell-intrinsic genetic deletion of Fcgr2b increased CD8+ effector T cell accumulation, resulting in accelerated graft rejection and decreased tumor volume in mouse models. Immunoglobulin G (IgG) antibody was not required for FcγRIIB-mediated control of CD8+ T cell immunity, and instead, the immunosuppressive cytokine fibrinogen-like 2 (Fgl2) was a functional ligand for FcγRIIB on CD8+ T cells. Fgl2 induced caspase-3/7-mediated apoptosis in Fcgr2b+, but not Fcgr2b-/-, CD8+ T cells. Increased expression of FcγRIIB correlated with freedom from rejection following withdrawal from immunosuppression in a clinical trial of kidney transplant recipients. Together, these findings demonstrate a cell-intrinsic coinhibitory function of FcγRIIB in regulating CD8+ T cell immunity.
Copyright © 2019. Published by Elsevier Inc.
Product Citations: 12
In Immunity on 14 January 2020 by Morris, A. B., Farley, C. R., et al.
-
Immunology and Microbiology
In Nature Cell Biology on 1 November 2019 by Jaumouillé, V., Cartagena-Rivera, A. X., et al.
αMβ2 integrin (complement receptor 3) is a major receptor for phagocytosis in macrophages. In other contexts, integrins' activities and functions are mechanically linked to actin dynamics through focal adhesions. We asked whether mechanical coupling of αMβ2 integrin to the actin cytoskeleton mediates phagocytosis. We found that particle internalization was driven by formation of Arp2/3 and formin-dependent actin protrusions that wrapped around the particle. Focal complex-like adhesions formed in the phagocytic cup that contained β2 integrins, focal adhesion proteins and tyrosine kinases. Perturbation of talin and Syk demonstrated that a talin-dependent link between integrin and actin and Syk-mediated recruitment of vinculin enable force transmission to target particles and promote phagocytosis. Altering target mechanical properties demonstrated more efficient phagocytosis of stiffer targets. Thus, macrophages use tyrosine kinase signalling to build a mechanosensitive, talin- and vinculin-mediated, focal adhesion-like molecular clutch, which couples integrins to cytoskeletal forces to drive particle engulfment.
-
ICC
-
Cell Biology
A protocol for the culture and isolation of murine synovial fibroblasts.
In Biomedical Reports on 1 August 2016 by Zhao, J., Ouyang, Q., et al.
The culture of synovial fibroblasts (SFs) is one of the most effective tools for investigating the pathology and physiology of synovial tissues and should prove useful for identifying the importance of SFs in disease as well as for the development of novel therapeutic approaches for several chronic joint diseases, such as rheumatoid arthritis. However, thus far, a detailed protocol for the primary culture and isolation of murine SFs has not been established. Therefore, the present study describes an easy and convenient method for isolating and culturing SFs from C57BL/6 mice. This protocol can be divided into 4 stages: Isolation of synovial tissues, isolation of SFs, seeding of SFs for growth in culture and purity analysis of SFs using the four cell markers, vimentin, cluster of differentiation 90.2 (CD90.2; Thy-1.2), intracellular adhesion molecule 1 (CD54) and vascular cell adhesion molecule 1 (CD106). This method is efficient and a purified population of SFs can be obtained 10 days after the initiation of culture.
Expression of nephronectin is inhibited by oncostatin M via both JAK/STAT and MAPK pathways.
In FEBS Open Bio on 24 April 2015 by Kurosawa, T., Yamada, A., et al.
Nephronectin (Npnt), also called POEM, is an extracellular matrix protein considered to play critical roles as an adhesion molecule in the development and functions of various tissues, such as the kidneys, liver, and bones. In the present study, we examined the molecular mechanism of Npnt gene expression and found that oncostatin M (OSM) strongly inhibited Npnt mRNA expression in MC3T3-E1 cells from a mouse osteoblastic cell line. OSM also induced a decrease in Npnt expression in both time- and dose-dependent manners via both the JAK/STAT and MAPK pathways. In addition, OSM-induced inhibition of osteoblast differentiation was recovered by over-expression of Npnt. These results suggest that OSM inhibits Npnt expression via the JAK/STAT and MAPK pathways, while down-regulation of Npnt by OSM influences inhibition of osteoblast differentiation.
-
FC/FACS
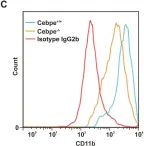
Human α-defensin expression is not dependent on CCAAT/enhancer binding protein-ε in a murine model.
In PLoS ONE on 25 March 2014 by Glenthøj, A., Dahl, S., et al.
Specific granule deficiency (SGD) is a rare congenital disorder characterized by recurrent infections. The disease is caused by inactivating mutations of the CCAAT/enhancer binding protein-ε (C/EBP-ε) gene. As a consequence, specific and gelatinase granules lack most matrix proteins. Furthermore, azurophil granules contain diminished amounts of their most abundant proteins, α-defensins, also known as human neutrophil peptides (HNPs). In accordance with this, in vitro models have demonstrated induction of HNPs by C/EBP-ε. Since mice do not express myeloid defensins, they cannot per se be used to characterize the role of C/EBP-ε in controlling HNP expression in vivo. We therefore crossed a transgenic HNP-1-expressing mouse with the Cebpe-/- mouse to study the in vivo significance of C/EBP-ε for HNP-1 transcription and expression. Surprisingly, neither expression nor processing of HNP-1 was affected by lack of C/EBP-ε in these mice. Transduction of C/EBP-ε into primary bone marrow cells from HNP-1 mice induced some HNP-1 expression, but not to levels comparable to expression human cells. Taken together, our data infer that the HNP-1 of the transgenic mouse does not show an expression pattern equivalent to endogenous secondary granule proteins. This limits the use of these transgenic mice as a model for human conditions.
-
FC/FACS
In PLoS One on 25 March 2014 by Glenthøj, A., Dahl, S., et al.
Fig.2.C

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1