There are numerous established techniques for isolating hepatic myeloid cells; however, preserving their phenotypic and functional characteristics can be challenging. We present a straightforward and efficient method to isolate hepatic myeloid cells, including Kupffer cells and lymphocyte antigen 6 complex, locus C+ (Ly6C+) monocytes/macrophages. The procedure involves perfusion of the liver with collagenase and purification with immunomagnetic particles. This protocol ensures the isolation of large quantities of purified, viable, and functional cells without influencing their physiological characteristics. For complete details on the use and execution of this protocol, please refer to Wu et al. (2019).1.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Product Citations: 27
In STAR Protocols on 15 December 2023 by Li, Y. T., Wu, H. L., et al.
A humanized orthotopic mouse model for preclinical evaluation of immunotherapy in Ewing sarcoma.
In Frontiers in Immunology on 23 October 2023 by Luo, W., Hoang, H., et al.
The advent of novel cancer immunotherapy approaches is revolutionizing the treatment for cancer. Current small animal models for most cancers are syngeneic or genetically engineered mouse models or xenograft models based on immunodeficient mouse strains. These models have been limited in evaluating immunotherapy regimens due to the lack of functional human immune system. Development of animal models for bone cancer faces another challenge in the accessibility of tumor engraftment sites. Here, we describe a protocol to develop an orthotopic humanized mouse model for a bone and soft tissue sarcoma, Ewing sarcoma, by transplanting fresh human cord blood CD34+ hematopoietic stem cells into young NSG-SGM3 mice combined with subsequent Ewing sarcoma patient derived cell engraftment in the tibia of the humanized mice. We demonstrated early and robust reconstitution of human CD45+ leukocytes including T cells, B cells, natural killer cells and monocytes. Ewing sarcoma xenograft tumors successfully orthotopically engrafted in the humanized mice with minimal invasive procedures. We validated the translational utility of this orthotopic humanized model by evaluating the safety and efficacy of an immunotherapy antibody, magrolimab. Treatment with magrolimab induces CD47 blockade resulting in significantly decreased primary tumor growth, decreased lung metastasis and prolonged animal survival in the established humanized model. Furthermore, the humanized model recapitulated the dose dependent toxicity associated with the CD47 blockade as observed in patients in clinical trials. In conclusion, this orthotopic humanized mouse model of Ewing sarcoma represents an improved platform for evaluating immunotherapy in bone and soft tissue sarcoma, such as Ewing sarcoma. With careful design and optimization, this model is generalizable for other bone malignancies.
Copyright © 2023 Luo, Hoang, Liao, Pan, Ayello and Cairo.
-
Immunology and Microbiology
Involucrin Modulates Vitamin D Receptor Activity in the Epidermis.
In The Journal of Investigative Dermatology on 1 June 2023 by Schmidt, A. D., Miciano, C., et al.
Terminally differentiated keratinocytes are critical for epidermal function and are surrounded by involucrin (IVL). Increased IVL expression is associated with a near-selective sweep in European populations compared with those in Africa. This positive selection for increased IVL in the epidermis identifies human adaptation outside of Africa. The functional significance is unclear. We hypothesize that IVL modulates the environmentally sensitive vitamin D receptor (VDR) in the epidermis. We investigated VDR activity in Ivl‒/‒ and wild-type mice using vitamin D agonist (MC903) treatment and comprehensively determined the inflammatory response using single-cell RNA sequencing and associated skin microbiome changes using 16S bacterial phylotyping. VDR activity and target gene expression were reduced in Ivl‒/‒ mouse skin, with decreased MC903-mediated skin inflammation and significant reductions in CD4+ T cells, basophils, macrophages, monocytes, and type II basal keratinocytes and an increase in suprabasal keratinocytes. Coinciding with the dampened MC903-mediated inflammation, the skin microbiota of Ivl‒/‒ mice was more stable than that of the wild-type mice, which exhibited an MC903-responsive increase in Bacteroidetes and a decrease in Firmicutes. Together, our studies in Ivl‒/‒ mice identify a functional role for IVL to positively impact VDR activity and suggest an emerging IVL/VDR paradigm for adaptation in the human epidermis.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
In Biology on 30 May 2023 by Masalova, O. V., Lesnova, E. I., et al.
Hepatitis C virus (HCV) is one of the basic culprits behind chronic liver disease, which may result in cirrhosis and hepatocarcinoma. In spite of the extensive research conducted, a vaccine against HCV has not been yet created. We have obtained human mesenchymal stem cells (hMSCs) and used them for expressing the HCV NS5A protein as a model vaccination platform. Sixteen hMSC lines of a different origin were transfected with the pcNS5A-GFP plasmid to obtain genetically modified MSCs (mMSCs). The highest efficiency was obtained by the transfection of dental pulp MSCs. C57BL/6 mice were immunized intravenously with mMSCs, and the immune response was compared with the response to the pcNS5A-GFP plasmid, which was injected intramuscularly. It was shown that the antigen-specific lymphocyte proliferation and the number of IFN-γ-synthesizing cells were two to three times higher after the mMSC immunization compared to the DNA immunization. In addition, mMSCs induced more CD4+ memory T cells and an increase in the CD4+/CD8+ ratio. The results suggest that the immunostimulatory effect of mMSCs is associated with the switch of MSCs to the pro-inflammatory phenotype and a decrease in the proportion of myeloid derived suppressor cells. Thus, the possibility of using human mMSCs for the creation of a vaccine against HCV has been shown for the first time.
-
FC/FACS
-
Genetics
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
In Nature Communications on 23 May 2023 by Yen, F. C., Glusac, J., et al.
With the increasing global demand for meat, cultured meat technologies are emerging, offering more sustainable solutions that aim to evade a future shortage of meat. Here, we demonstrate a cultured meat platform composed of edible microcarriers and an oleogel-based fat substitute. Scalable expansion of bovine mesenchymal stem cells on edible chitosan-collagen microcarriers is optimized to generate cellularized microtissues. In parallel, an oleogel system incorporated with plant protein is developed as a fat substitute, which is comparable to beef fat in appearance and texture. Combining the cellularized microtissues with the developed fat substitute, two types of cultured meat prototypes are introduced: layered cultured meat and burger-like cultured meat. While the layered prototype benefits enhanced stiffness, the burger-like prototype has a marbling meat-like appearance and a softer texture. Overall, this platform and the established technological basis may contribute to the development of different cultured meat products and promote their commercial production.
© 2023. The Author(s).
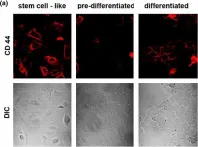
In Breast Cancer Res on 12 May 2009 by Williams, C., Helguero, L., et al.
Fig.3.A

-
ICC-IF
-
Collected and cropped from Breast Cancer Res by CiteAb, provided under a CC-BY license
Image 1 of 1