Spinal muscular atrophy (SMA) is characterized by low levels of the ubiquitously expressed Survival Motor Neuron (SMN) protein, leading to progressive muscle weakness and atrophy. Skeletal muscle satellite cells play a crucial role in muscle fiber maintenance, repair, and remodelling. While the effects of SMN depletion in muscle are well documented, its precise role in satellite cell function remains largely unclear. Using the Smn2B/- mouse model, we investigated SMN-depleted satellite cell biology through single fiber culture studies. Myofibers from Smn2B/- mice were smaller in size, shorter in length, had reduced myonuclear domain size, and reduced sub-synaptic myonuclear clusters-all suggesting impaired muscle function and integrity. These changes were accompanied by a reduction in the number of myonuclei in myofibers from Smn2B/- mice across all disease stages examined. Although the number of satellite cells in myofibers was significantly reduced, those remaining retained their capacity for myogenic activation and proliferation. These findings support the idea that a dysregulated myogenic process could be occurring as early in muscle stem cells during muscle formation and maturation in SMA. Targeting those pathways could offer additional options for combinatorial therapies for SMA.
© The Author(s) 2024. Published by Oxford University Press.
Product Citations: 154
SMN depletion impairs skeletal muscle formation and maturation in a mouse model of SMA.
In Human Molecular Genetics on 23 January 2025 by Liu, H., Chehade, L., et al.
-
Genetics
E-box independent chromatin recruitment turns MYOD into a transcriptional repressor
Preprint on BioRxiv : the Preprint Server for Biology on 5 December 2024 by Nicoletti, C., Massenet, J., et al.
MYOD is an E-box sequence-specific basic Helix-Loop-Helix (bHLH) transcriptional activator that, when expressed in non-muscle cells, induces nuclear reprogramming toward skeletal myogenesis by promoting chromatin accessibility at previously silent loci. Here, we report on the identification of a previously unrecognized property of MYOD as repressor of gene expression, via E-box-independent chromatin binding within accessible genomic elements, which invariably leads to reduced chromatin accessibility. MYOD-mediated repression requires the integrity of functional domains previously implicated in MYOD-mediated activation of gene expression. Repression of mitogen-and growth factor-responsive genes occurs through promoter binding and requires a highly conserved domain within the first helix. Repression of cell-of-origin/alternative lineage genes occurs via binding and decommissioning of distal regulatory elements, such as super-enhancers (SE), which requires the N-terminal activation domain as well as two chromatin-remodeling domains and leads to reduced strength of CTCF-mediated chromatin interactions. Surprisingly, MYOD-mediated chromatin compaction and repression of transcription do not associate with reduction of H3K27ac, the conventional histone mark of enhancer or promoter activation, but with reduced levels of the recently discovered histone H4 acetyl-methyl lysine modification (Kacme). These results extend MYOD biological properties beyond the current dogma that restricts MYOD function to a monotone transcriptional activator and reveal a previously unrecognized functional versatility arising from an alternative chromatin recruitment through E-box or non-E-box sequences. The E-box independent repression of gene expression by MYOD might provide a promiscuous mechanism to reduce chromatin accessibility and repress cell-of-origin/alternative lineage and growth factor/mitogen-responsive genes to safeguard the integrity of cell identity during muscle progenitor commitment toward the myogenic lineage.
-
Biochemistry and Molecular biology
In Molecular Medicine Reports on 1 November 2024 by Liu, H. & Joung, H.
Ginkgolic acid (GA), isolated from the leaves and seed coats of Ginkgo biloba, exerts several biological effects, including antitumor, antibacterial, anti‑HIV and anti‑inflammatory effects. However, the effects of GA on C2C12 myoblasts remain unclear. The present study assessed cell viability with the MTT assay and evaluated colony formation through crystal violet staining. Flow cytometry was used to analyze apoptosis with Annexin V/7‑AAD staining, proliferation with Ki67 staining and cell cycle arrest. Western blotting detected myogenic markers and other relevant proteins. Myotube formation was examined by immunofluorescence, and autophagy was measured using an LC3 antibody‑based kit via flow cytometry. The present study showed that treatment of C2C12 cells with GA significantly inhibited their viability and colony formation capacity but did not trigger apoptosis, as indicated by Annexin V/7‑AAD staining. However, Ki67 staining indicates that GA exerted dose‑dependent antiproliferative effects. Further analysis revealed that GA partially inhibited the growth of C2C12 cells via cell cycle arrest in S phase, highlighting its role in the disruption of cell proliferation. Furthermore, treatment with GA impaired myoblast differentiation, as evidenced by a reduction in the expression of the myogenesis markers, the myosin‑heavy chain, myoblast determination protein 1 and myogenin, and suppressed myotube formation. Notably, during C2C12 cell differentiation, GA promoted apoptosis without affecting cell cycle progression or Ki67 expression. Mechanistically, GA could suppress nuclear extracellular signal‑regulated kinase phosphorylation, suggesting that it modulates cell proliferation pathways. Moreover, GA triggered autophagy in differentiated C2C12 cells, as confirmed by elevated LC3 II levels. These findings highlight the multifaceted effects of GA on C2C12 cells.
-
WB
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
Satellite cell-derived TRIM28 is pivotal for mechanical load- and injury-induced myogenesis.
In EMBO Reports on 1 September 2024 by Lin, K. H., Hibbert, J. E., et al.
Satellite cells are skeletal muscle stem cells that contribute to postnatal muscle growth, and they endow skeletal muscle with the ability to regenerate after a severe injury. Here we discover that this myogenic potential of satellite cells requires a protein called tripartite motif-containing 28 (TRIM28). Interestingly, different from the role reported in a previous study based on C2C12 myoblasts, multiple lines of both in vitro and in vivo evidence reveal that the myogenic function of TRIM28 is not dependent on changes in the phosphorylation of its serine 473 residue. Moreover, the functions of TRIM28 are not mediated through the regulation of satellite cell proliferation or differentiation. Instead, our findings indicate that TRIM28 regulates the ability of satellite cells to progress through the process of fusion. Specifically, we discover that TRIM28 controls the expression of a fusogenic protein called myomixer and concomitant fusion pore formation. Collectively, the outcomes of this study expose the framework of a novel regulatory pathway that is essential for myogenesis.
© 2024. The Author(s).
In Frontiers in Cell and Developmental Biology on 1 March 2024 by Xie, N., Robinson, K., et al.
Producing an adequate number of muscle stem cells (MuSCs) with robust regenerative potential is essential for the successful cell therapy of muscle-wasting disorders. We have recently developed a method to produce skeletal myogenic cells with exceptional engraftability and expandability through an in vivo pluripotent stem cell (PSC) differentiation approach. We have subsequently mapped engraftment and gene expression and found that leukemia inhibitory factor receptor (Lifr) expression is positively correlated with engraftability. We therefore investigated the effect of LIF, the endogenous ligand of LIFR, on cultured MuSCs and examined their engraftment potential. We found that LIF-treated MuSCs exhibited elevated expression of PAX7, formed larger colonies from single cells, and favored the retention of PAX7+ "reserve cells" upon myogenic differentiation. This suggested that LIF promoted the maintenance of cultured MuSCs at a stem cell stage. Moreover, LIF enhanced the engraftment capability of MuSCs that had been expanded in vitro for 12 days by 5-fold and increased the number of MuSCs that repopulated the stem cell pool post-transplantation. These results thereby demonstrated the effectiveness of our in vivo PSC differentiation platform to identify positive regulators of the engraftability of cultured MuSCs.
Copyright © 2024 Xie, Robinson, Sundquist and Chan.
-
Stem Cells and Developmental Biology
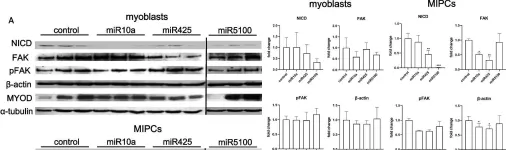
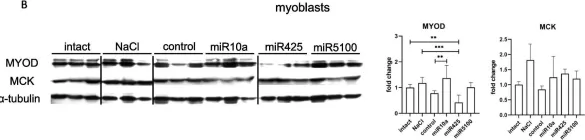
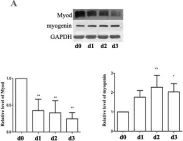
In Stem Cell Res Ther on 15 August 2023 by Mierzejewski, B., Grabowska, I., et al.
Fig.4.A

-
WB
-
Collected and cropped from Stem Cell Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 12
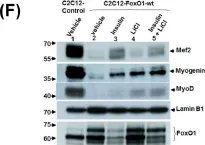
In Stem Cell Res Ther on 15 August 2023 by Mierzejewski, B., Grabowska, I., et al.
Fig.8.B

-
WB
-
Collected and cropped from Stem Cell Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 12
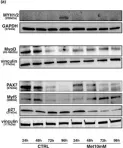
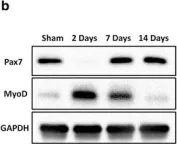
In PLoS One on 11 February 2023 by Maniscalco, E., Abbadessa, G., et al.
Fig.4.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 12
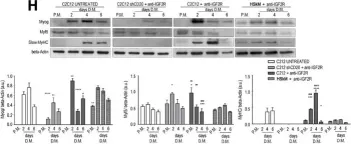
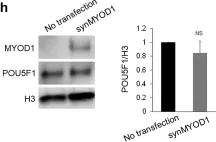
In EMBO Mol Med on 9 January 2020 by Bella, P., Farini, A., et al.
Fig.2.H
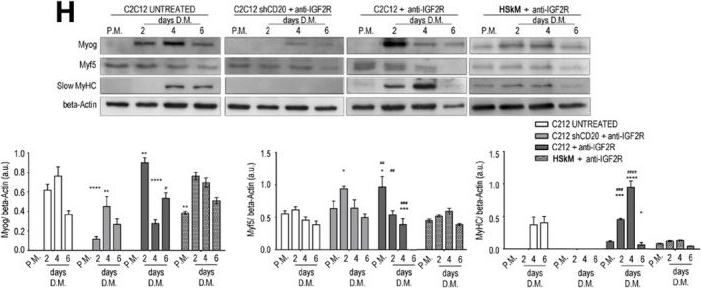
-
WB
-
Mus musculus (House mouse)
Collected and cropped from EMBO Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 12
In PLoS One on 12 October 2018 by Hellbach, N., Peterson, S., et al.
Fig.2.A

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 12
In Sci Rep on 19 January 2018 by Akiyama, T., Sato, S., et al.
Fig.2.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 12
In Sci Rep on 19 January 2018 by Akiyama, T., Sato, S., et al.
Fig.1.H

-
WB
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 12
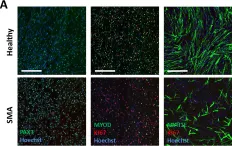
In Nat Commun on 9 January 2018 by Rao, L., Qian, Y., et al.
Fig.2.C

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 12
In Stem Cell Res Ther on 17 October 2017 by Yousuf, Y., Jeschke, M. G., et al.
Fig.5.B

-
WB
-
Collected and cropped from Stem Cell Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 12
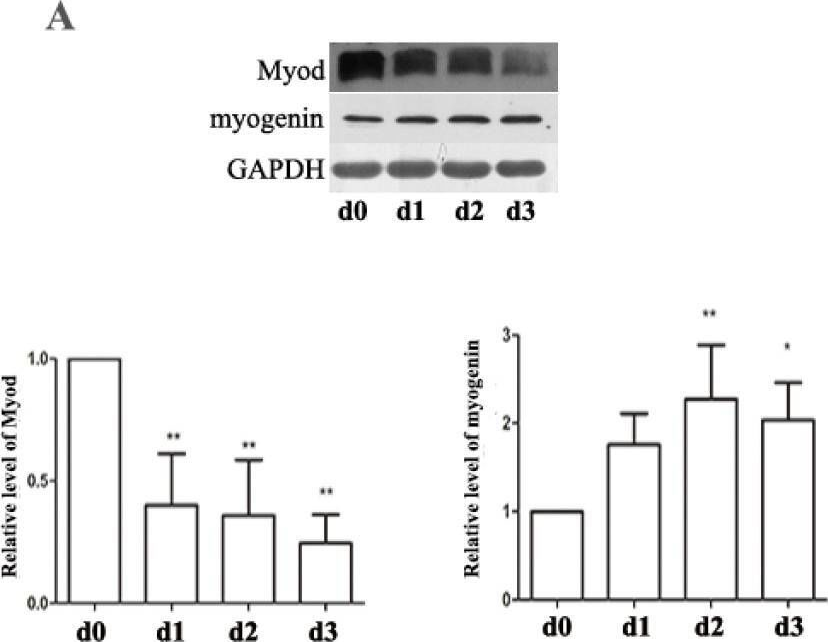
In Nutrients on 8 May 2015 by Dai, J. M., Yu, M. X., et al.
Fig.5.C

-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from Nutrients by CiteAb, provided under a CC-BY license
Image 1 of 12
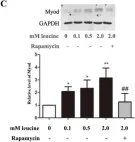
In Nutrients on 8 May 2015 by Dai, J. M., Yu, M. X., et al.
Fig.5.A
-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from Nutrients by CiteAb, provided under a CC-BY license
Image 1 of 12
In PLoS One on 20 February 2014 by Wu, Y. J., Fang, Y. H., et al.
Fig.3.F
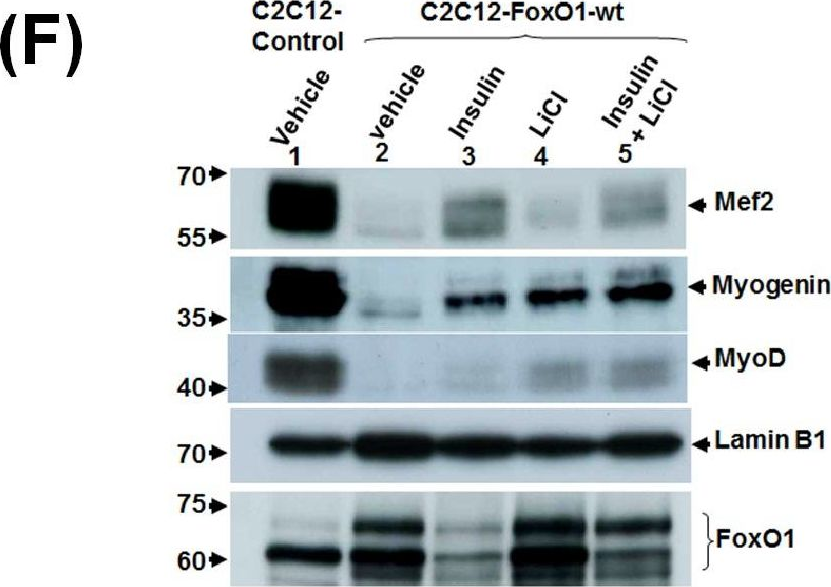
-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 12