Head and neck cancer (HNC) is common worldwide. Given poor outcomes for patients with HNC, research into targeted therapies is needed. Ataxia telangiectasia mutated (ATM) is a DNA damage kinase which is activated by double-strand DNA breaks. We tested the effects of a novel ATM inhibitor on HNC cell lines and xenografts.
p53-Binding protein 1 and phosphorylated ATM were localized in cultured cells by immunofluorescence microscopy. Protein expression was determined by western blot. Tumor xenografts were established by injecting HNC lines into immunocompromised mice. Tumor sections were characterized by immunohistochemistry. Apoptotic cells were determined by terminal transferase-mediated dUTP nick-end labeling assay.
ATM inhibition increased double-strand DNA breaks at replication foci in HNC cell lines. ATM inhibition affected cell-cycle regulatory protein expression, blocked cell-cycle progression at the G2/M phase and resulted in apoptosis.
ATM inhibition may be therapeutically useful in treating HNC.
Copyright © 2021 International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Product Citations: 8
DNA Double-strand Break Signaling Is a Therapeutic Target in Head and Neck Cancer.
In Anticancer Research on 1 November 2021 by Wu, J., Galvan, K. J., et al.
-
Cancer Research
-
Genetics
In Scientific Reports on 18 September 2019 by Lee, S. R., Quan, K. T., et al.
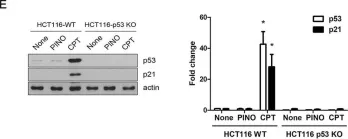
Plant-derived lignans have numerous biological effects including anti-tumor and anti-inflammatory activities. Screening of purified constituents of Rubia philippinensis from human glioblastoma cells resistant to TNF-related apoptosis-inducing ligand (TRAIL) has suggested that the lignan pinoresinol was a highly active TRAIL sensitizer. Here we show that treatment with nontoxic doses of pinoresinol in combination with TRAIL induced rapid apoptosis and caspase activation in many types of glioblastoma cells, but not in normal astrocytes. Analyses of apoptotic signaling events revealed that pinoresinol enhanced the formation of TRAIL-mediated death-inducing signaling complex (DISC) and complete processing of procaspase-8 within the DISC in glioblastoma cells, in which caspase-8 was inactivated. Mechanistically, pinoresinol downregulated the expression of cellular FLICE-inhibitory protein (cFLIPL) and survivin through proteasome-mediated degradation, without affecting death receptors or downstream intracellular apoptosis-related proteins. Furthermore, the sensitization of TRAIL-mediated apoptosis by pinoresinol strictly depended on the expression level of cFLIPL, which was regulated through de novo protein synthesis, rather than by NF-κB or p53 signaling. Taken together, our results indicate that pinoresinol facilitates DISC-mediated caspase-8 activation by targeting cFLIPL in an early event in apoptotic signaling, which provides a potential therapeutic module for TRAIL-based chemotherapy.
-
WB
PRMT1-Mediated Translation Regulation Is a Crucial Vulnerability of Cancer.
In Cancer Research on 1 September 2017 by Hsu, J. H., Hubbell-Engler, B., et al.
Through an shRNA screen, we identified the protein arginine methyltransferase Prmt1 as a vulnerable intervention point in murine p53/Rb-null osteosarcomas, the human counterpart of which lacks effective therapeutic options. Depletion of Prmt1 in p53-deficient cells impaired tumor initiation and maintenance in vitro and in vivo Mechanistic studies reveal that translation-associated pathways were enriched for Prmt1 downstream targets, implicating Prmt1 in translation control. In particular, loss of Prmt1 led to a decrease in arginine methylation of the translation initiation complex, thereby disrupting its assembly and inhibiting translation. p53/Rb-null cells were sensitive to p53-induced translation stress, and analysis of human cancer cell line data from Project Achilles further revealed that Prmt1 and translation-associated pathways converged on the same functional networks. We propose that targeted therapy against Prmt1 and its associated translation-related pathways offer a mechanistic rationale for treatment of osteosarcomas and other cancers that exhibit dependencies on translation stress response. Cancer Res; 77(17); 4613-25. ©2017 AACR.
©2017 American Association for Cancer Research.
-
Biochemistry and Molecular biology
-
Cancer Research
High glucose induces the aging of mesenchymal stem cells via Akt/mTOR signaling.
In Molecular Medicine Reports on 1 August 2017 by Zhang, D., Lu, H., et al.
It has previously been demonstrated that glucose is important in the process of stem cell aging. However, the mechanisms of cell senescence induced by high glucose (HG) remain to be elucidated. The preliminary study indicated that D‑galactose induced mesenchymal stem cell (MSCs) aging. The present study demonstrated, following treatment with 11.0 or 22.0 mM HG for 14 days, that HG significantly promoted MSCs aging and the expression levels of phosphorylated (p-)phosphatidylinositol 3-kinase/protein kinase B (Akt) and p‑mammalian target of rapamycin signaling (mTOR) in the HG groups were increased compared with the control group. However, following Akt inhibition with 1.0 or 10.0 nM MK‑2206, which is an Akt‑specific small molecule inhibitor, the senescence‑cell value in the HG group was significantly decreased compared with the control group. These results indicated that HG induced MSCs senescence and this effect was primarily mediated via the Akt/mTOR signaling pathway.
-
WB
-
Rattus norvegicus (Rat)
-
Biochemistry and Molecular biology
-
Stem Cells and Developmental Biology
In Stroke; A Journal of Cerebral Circulation on 1 February 2009 by Niizuma, K., Endo, H., et al.
p53-upregulated modulator of apoptosis (PUMA), a BH3-only member of the Bcl-2 protein family, is required for p53-dependent and -independent forms of apoptosis. PUMA localizes to mitochondria and interacts with antiapoptotic Bcl-2 and Bcl-X(L) or proapoptotic Bax in response to death stimuli. Although studies have shown that PUMA is associated with pathomechanisms of cerebral ischemia, clearly defined roles for PUMA in ischemic neuronal death remain unclear. The purpose of this study was to determine potential roles for PUMA in cerebral ischemia.
Five minutes of transient global cerebral ischemia (tGCI) were induced by bilateral common carotid artery occlusion combined with hypotension.
PUMA was upregulated in vulnerable hippocampal CA1 neurons after tGCI as shown by immunohistochemistry. In Western blot and coimmunoprecipitation analyses, PUMA localized to mitochondria and was bound to Bcl-X(L) and Bax in the hippocampal CA1 subregion after tGCI. PUMA upregulation was inhibited by pifithrin-alpha, a specific inhibitor of p53, suggesting that PUMA is partly controlled by the p53 transcriptional pathway after tGCI. Furthermore, reduction in oxidative stress by overexpression of copper/zinc superoxide dismutase, which is known to be protective of vulnerable ischemic hippocampal neurons, inhibited PUMA upregulation and subsequent hippocampal CA1 neuronal death after tGCI.
These results imply a potential role for PUMA in delayed CA1 neuronal death after tGCI and that it could be a molecular target for therapy.
-
WB
-
Rattus norvegicus (Rat)
-
Cardiovascular biology
-
Neuroscience
In Sci Rep on 18 September 2019 by Lee, S. R., Quan, K. T., et al.
Fig.3.E

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 1