This study investigates age-related neurodegeneration in the cerebellar cortex, emphasizing the role of IL-6 deficiency in preserving Purkinje cells. We found that apoptosis plays a minimal role in Purkinje cell loss by using 4-month- and 24-month-old wild-type (WT) and IL-6 knockout (IL-6KO) mice. At 24 months, WT mice exhibited severe Purkinje cell degeneration, including atrophic cell bodies, eosinophilic cytoplasm, pyknotic nuclei, mitochondrial disruption, and increased levels of lipofuscin-rich lysosomes. In contrast, IL-6KO mice showed fewer lysosomes, reduced mitochondrial damage, and less neuronal atrophy, indicating a neuroprotective effect. Lower p53 expression and decreased levels of its downstream effectors (p21, and Bax) in IL-6KO mice correlated with reduced cellular stress. Minimal changes in apoptotic markers (Bax and caspase-3) further reinforce the limited role of apoptosis. Neuroinflammation, marked by elevated GFAP, was prominent in aged WT mice but attenuated in IL-6KO mice. Reduced p53 accumulation, less severe neuroinflammation, and preserved metabolic homeostasis in IL-6KO mice correlated with improved Purkinje cell survival. These findings suggest that IL-6 accelerates neurodegeneration via p53-associated stress and inflammation, while IL-6 deficiency mitigates these effects. Targeting IL-6 signaling through anti-inflammatory strategies or IL-6 inhibition may offer a therapeutic approach for age-related neurodegenerative disorders.
Product Citations: 6
In Cells on 2 April 2025 by Cieślińska, M. W., Bialuk, I., et al.
-
Cell Biology
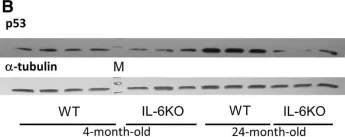
IL-6 deficiency attenuates p53 protein accumulation in aged male mouse hippocampus.
In Biogerontology on 1 February 2020 by Bialuk, I., Cieslinska, M., et al.
Our earlier studies demonstrated slower age-related memory decline in IL-6-deficient than in control mice. Therefore, in the present study we evaluated the effect of IL-6 deficiency and aging on expression of p53, connected with accumulation of age-related cellular damages, in hippocampus of 4- and 24-month-old IL-6-deficient C57BL/6J (IL-6KO) and wild type control (WT) mice. The accumulation of p53 protein in hippocampus of aged IL-6KO mice was significantly lower than in aged WT ones, while p53 mRNA level was significantly higher in IL-6-deficient mice, what indicates that the effect was independent on p53 transcription. Presence of few apoptotic cells in hippocampal dentate gyrus and lack of changes in levels of pro-apoptotic Bax, antiapoptotic Bcl-2, as well as in p21 protein in aged animals of both genotypes, points to low transcriptional activity of p53, especially in aged WT mice. Because the amount of p53 protein did not correlate with the level of Mdm2 protein, its main negative regulator, other than Mdm2-dependent mechanism was involved in p53 build-up. Significantly higher mRNA levels of autophagy-associated genes: Pten, Tsc2, and Dram1 in IL-6KO mice, in conjunction with significantly lower amount of Bcl-2 protein in 4-month-old IL-6KO mice, suggests that lack of IL-6/STAT3/Bcl-2 signaling could account for better autophagy performance in these mice, preventing excessive accumulation of proteins. Taken together, attenuated p53 protein build-up, absence of enhanced apoptosis, and transcriptional up-regulation of autophagy-associated genes imply that IL-6 deficiency may protect hippocampus from age-related accumulation of cellular damages.
-
WB
-
Mus musculus (House mouse)
-
Neuroscience
Glucose-6-phosphate dehydrogenase is critical for suppression of cardiac hypertrophy by H2S.
In Cell Death Discovery on 1 December 2018 by Chhabra, A., Mishra, S., et al.
Hydrogen Sulfide (H2S), recently identified as the third endogenously produced gaseous messenger, is a promising therapeutic prospect for multiple cardio-pathological states, including myocardial hypertrophy. The molecular niche of H2S in normal or diseased cardiac cells is, however, sparsely understood. Here, we show that β-adrenergic receptor (β-AR) overstimulation, known to produce hypertrophic effects in cardiomyocytes, rapidly decreased endogenous H2S levels. The preservation of intracellular H2S levels under these conditions strongly suppressed hypertrophic responses to adrenergic overstimulation, thus suggesting its intrinsic role in this process. Interestingly, unbiased global transcriptome sequencing analysis revealed an integrated metabolic circuitry, centrally linked by NADPH homeostasis, as the direct target of intracellular H2S augmentation. Within these gene networks, glucose-6-phosphate dehydrogenase (G6PD), the first and rate-limiting enzyme (producing NADPH) in pentose phosphate pathway, emerged as the critical node regulating cellular effects of H2S. Utilizing both cellular and animal model systems, we show that H2S-induced elevated G6PD activity is critical for the suppression of cardiac hypertrophy in response to adrenergic overstimulation. We also describe experimental evidences suggesting multiple processes/pathways involved in regulation of G6PD activity, sustained over extended duration of time, in response to endogenous H2S augmentation. Our data, thus, revealed H2S as a critical endogenous regulator of cardiac metabolic circuitry, and also mechanistic basis for its anti-hypertrophic effects.
-
WB
-
Rattus norvegicus (Rat)
-
Cardiovascular biology
In Cell Death & Disease on 6 March 2018 by Cai, B., Ma, M., et al.
The proliferation, apoptosis, and differentiation of myoblasts are essential processes in skeletal muscle development. During this developmental process, microRNAs (miRNAs) play crucial roles. In our previous RNA-seq study (accession number GSE62971), we found that miR-16-5p was differentially expressed between fast and slow growth in chicken. In this study, we report that miR-16-5p could inhibit myoblast proliferation, promote myoblast apoptosis, and repress myoblast differentiation by directly binding to the 3' UTR of SESN1, which is also differentially expressed. Overexpression of SESN1 significantly promoted the proliferation, inhibited apoptosis, and induced differentiation of myoblasts. Conversely, its loss of function hampered myoblast proliferation, facilitated myoblast apoptosis, and inhibited myoblast differentiation. Interestingly, we found SESN1 could regulate p53 by a feedback mechanism, thereby participating in the regulation of p53 signaling pathway, which suggests that this feedback is indispensable for myoblast proliferation and apoptosis. Altogether, these data demonstrated that miR-16-5p directly targets SESN1 to regulate the p53 signaling pathway, and therefore affecting myoblast proliferation and apoptosis. Additionally, SESN1 targets myogenic genes to control myoblast differentiation.
-
WB
-
Cell Biology
p16, pRb, and p53 in Feline Oral Squamous Cell Carcinoma.
In Veterinary Sciences on 18 August 2016 by Supsavhad, W., Dirksen, W. P., et al.
Feline oral squamous cell carcinoma (FOSCC) is a highly aggressive head and neck cancer in cats, but the molecular pathogenesis of this cancer is still uncertain. In this study, p16, p53, and pRb proteins were detected and quantified by immunohistochemistry in forty-three FOSCC primary tumors and three FOSCC xenografts. p16 mRNA levels were also measured in three FOSCC cell lines (SCCF1, F2, and F3), which were consistent with their p16 immunoreactivity. Feline SCCF1 cells had very high levels of p16 protein and mRNA (55-fold greater) compared to SCCF2 and F3. A partial feline p16 cDNA sequence was amplified and sequenced. The average age of cats with FOSCC with high p16 immunoreactivity was significantly lower than the average age in the low p16 group. Eighteen of 43 (42%) FOSCCs had low p16 intensity, while 6/43 (14%) had high p16 immunoreactivity. Feline papillomavirus L1 (major capsid) DNA was not detected in the SCC cell lines or the FOSCCs with high p16 immunostaining. Five of 6 (83%) of the high p16 FOSCC had low p53, but only 1/6 (17%) had low pRb immunoreactivity. In summary, the staining pattern of p16, p53, and pRb in FOSCC was different from human head and neck squamous cell carcinoma and feline cutaneous squamous cell carcinoma. The majority of FOSCCs have low p16 immunostaining intensity, therefore, inactivation of CDKN2A is suspected to play a role in the pathogenesis of FOSCC. A subset of FOSCCs had increased p16 protein, which supports an alternate pathogenesis of cancer in these cats.
-
Cancer Research
-
Veterinary Research
In Biogerontology on 1 February 2020 by Bialuk, I., Cieslinska, M., et al.
Fig.1.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Biogerontology by CiteAb, provided under a CC-BY license
Image 1 of 3
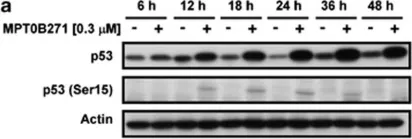
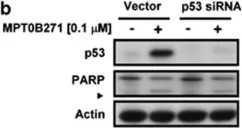
In Cell Death Dis on 10 April 2014 by Tsai, A. C., Wang, C. Y., et al.
Fig.5.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 3
In Cell Death Dis on 10 April 2014 by Tsai, A. C., Wang, C. Y., et al.
Fig.5.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 3