Influenza virus infection is a significant cause of global mortality. However, the development of influenza vaccines that induce robust immune responses at the site of respiratory mucosal exposure has proven challenging. Here, we assessed immune responses and protective efficacy of a rhesus adenovirus serotype 52 (RhAd52) vectored influenza vaccine encoding the hemagglutinin (HA) glycoprotein from A/California/07/2009 administrated by systemic or mucosal routes of immunization. We observed robust and durable systemic and mucosal immunity, including IgA and tissue resident memory T cells in the respiratory mucosa, in mice that received the vaccine intranasally or intratracheally. In contrast, only systemic immune responses were observed in mice that received the vaccine intramuscularly. Moreover, a single intranasal or intratracheal dose of RhAd52-HA provided near complete protection against a lethal challenge with a mouse-adapted influenza virus strain, whereas intramuscular immunization with RhAd52-HA and mRNA-HA provided less robust protection. Our data demonstrate the importance of mucosal immunity for enhancing vaccine protection against influenza.
© 2025 The Authors.
Product Citations: 364
Mucosal boosting increases protective efficacy of an influenza vaccine in mice.
In IScience on 20 June 2025 by Wang, L., Chan, C. N., et al.
-
Immunology and Microbiology
In IScience on 16 May 2025 by Farías, M. A., Cancino, F. A., et al.
Herpes simplex virus type 1 (HSV-1) significantly impairs dendritic cell (DC) function, ultimately eliciting the death of these cells. Here, we sought to assess whether HSV-1 modulates lipid metabolism in mouse DCs as a mechanism of immune evasion. For this, we performed RT-qPCR gene arrays with ingenuity pathway analysis (IPA), RNA sequencing (RNA-seq) and gene set enrichment analysis (GSEA), confocal microscopy, transmission electron microscopy, ultra-high-performance liquid chromatography-quadrupole time-of-flight (UHPLC-QTOF) analysis, pharmacological inhibition of eight lipid-metabolism-related enzymes in HSV-1-infected DCs, co-cultures between virus-specific transgenic CD4+ and CD8+ T cells and HSV-1-infected DCs, and in vivo assays with mice. We found that HSV-1 significantly alters lipid metabolism in DCs and induces lipid droplet (LD) accumulation in these cells. Pharmacological inhibition of two particular lipid metabolism enzymes was found to partially restore DC function. Overall, these results suggest that lipid metabolism plays an important role in the impairment of DC function by HSV-1.
© 2025 The Authors.
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
Dysfunctional β-cell autophagy induces β-cell stress and enhances islet immunogenicity.
In Frontiers in Immunology on 13 February 2025 by Austin, M. C., Muralidharan, C., et al.
Type 1 Diabetes (T1D) is caused by a combination of genetic and environmental factors that trigger autoimmune-mediated destruction of pancreatic β-cells. Defects in β-cell stress response pathways such as autophagy may play an important role in activating and/or exacerbating the immune response in disease development. Previously, we discovered that β-cell autophagy is impaired prior to the onset of T1D, implicating this pathway in T1D pathogenesis.
To assess the role of autophagy in β-cell health and survival, and whether defects in autophagy render islets more immunogenic.
We knocked out the critical autophagy enzyme, ATG7, in the β-cells of mice (ATG7Δβ-cell) then monitored blood glucose, performed glucose tolerance tests, and evaluated bulk islet mRNA and protein. We also assessed MHC-I expression and presence of CD45+ immune cells in ATG7Δβ-cell islets and evaluated how impaired autophagy affects EndoC-βH1 HLA-I expression under basal and IFNα stimulated conditions. Lastly, we co-cultured ATG7Δβ-cell islet cells with diabetogenic BDC2.5 helper T cells and evaluated T cell activation.
We found that all ATG7Δβ-cell mice developed diabetes between 11-15 weeks of age. Gene ontology analysis revealed a significant upregulation of pathways involved in inflammatory processes, response to ER stress, and the ER-associated degradation pathway. Interestingly, we also observed upregulation of proteins involved in MHC-I presentation, suggesting that defective β-cell autophagy may alter the immunopeptidome, or antigen repertoire, and enhance β-cell immune visibility. In support of this hypothesis, we observed increased MHC-I expression and CD45+ immune cells in ATG7Δβ-cell islets. We also demonstrate that HLA-I is upregulated in EndoC β-cells when autophagic degradation is inhibited. This effect was observed under both basal and IFNα stimulated conditions. Conversely, a stimulator of lysosome acidification/function, C381, decreased HLA-I expression. Lastly, we showed that in the presence of islet cells with defective autophagy, there is enhanced BDC2.5 T cell activation.
Our findings demonstrate that β-cell autophagy is critical to cell survival/function. Defective β-cell autophagy induces ER stress, alters pathways of antigen production, and enhances MHC-I/HLA-I presentation to surveilling immune cells. Overall, our results suggest that defects in autophagy make β-cells more susceptible to immune attack and destruction.
Copyright © 2025 Austin, Muralidharan, Roy, Crowder, Piganelli and Linnemann.
-
Mus musculus (House mouse)
-
Cell Biology
-
Immunology and Microbiology
In IScience on 17 January 2025 by Matsushima, R., Wakamatsu, E., et al.
A co-signaling receptor, 2B4, has dual effects in immune cells, but its actual functions in T cells remain elusive. Here, using super-resolution imaging technology with an immunological synapse model, we showed that 2B4 forms "2B4 microclusters" immediately after 2B4-CD48 binding. A lipid phosphatase, SHIP-1, subsequently combined with 2B4 to form coinhibitory signalosomes, leading to the suppression of cytokine production. An activating adapter, SLAM-associated protein (SAP), attenuated the clustering of SHIP-1 and recruited a kinase, Fyn, enhancing the Vav1 signaling pathway as costimulatory signalosomes. Furthermore, we found that a chimeric antigen receptor with a 2B4 tail (2B4-CAR) retained the original signal transduction mechanism of 2B4. With endogenous levels of SAP expression, 2B4-CAR-T cells exposed sufficient antitumor efficacy in vivo without excess cytokine production. Our results may help explain the biphasic feature of 2B4 in T cell responses from the viewpoint of the signalosome and provide a new candidate for CAR development.
© 2024 The Authors.
-
Immunology and Microbiology
In Nature Communications on 14 January 2025 by Hawman, D. W., Tipih, T., et al.
The ongoing circulation of influenza A H5N1 in the United States has raised concerns of a pandemic caused by highly pathogenic avian influenza. Although the United States has stockpiled and is prepared to produce millions of vaccine doses to address an H5N1 pandemic, currently circulating H5N1 viruses contain multiple mutations within the immunodominant head domain of hemagglutinin (HA) compared to the antigens used in stockpiled vaccines. It is unclear if these stockpiled vaccines will need to be updated to match the contemporary H5N1 strains. Here we show that a replicating RNA vaccine expressing the HA of an H5N1 isolated from a US dairy cow confers complete protection against homologous lethal challenge in mice. A repRNA encoding the HA of a clade 1 H5 from 2004 (A/Vietnam/1203/2004) as utilized by some stockpiled vaccines, confers only partial protection. Our data highlight the utility of nucleic acid vaccines to be rapidly updated to match emergent viruses of concern while demonstrating that contemporary bovine H5N1 viruses can evade immunity elicited by historical HA antigens.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
-
Mus musculus (House mouse)
-
Genetics
-
Immunology and Microbiology
-
Veterinary Research
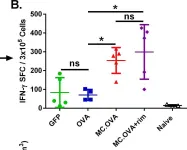
In PLoS One on 16 October 2016 by Collinson-Pautz, M. R., Slawin, K. M., et al.
Fig.4.B

-
ELISpot
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3
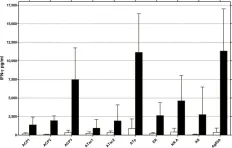
In PLoS Negl Trop Dis on 7 January 2014 by Roupie, V., Pidot, S. J., et al.
Fig.2.A

-
ELISA
-
Mus musculus (House mouse)
Collected and cropped from PLoS Negl Trop Dis by CiteAb, provided under a CC-BY license
Image 1 of 3
In PLoS Negl Trop Dis on 7 January 2014 by Roupie, V., Pidot, S. J., et al.
Fig.4.A

-
ELISA
-
Mus musculus (House mouse)
Collected and cropped from PLoS Negl Trop Dis by CiteAb, provided under a CC-BY license
Image 1 of 3