Interferon regulatory factor 4 (IRF4) is a critical transcription factor that governs the differentiation of cluster of differentiation 4+ (CD4+) T cells. The pathogenesis and progression of psoriasis are primarily attributed to an immune imbalance stemming from the overproduction of interleukin-17A (IL-17A) by T lymphocytes. However, the role of IRF4 in psoriasis remains unexplored. In this study, we found that IRF4 activity is increased in the cutaneous lesions of patients with psoriasis in response to stimulation by IL-23A and IL-1β. This IRF4 elevation heightens its binding to the E1A binding protein p300 (EP300) promoter, triggering the transcription of downstream retinoic acid receptor-related orphan receptor-γt (RORγt) and increasing the secretion of IL-17A, thereby establishing the IL-1β/IL-23A-IRF4-EP300-RORC-IL-17A inflammatory cascade in psoriasis. The alleviation of imiquimod (IMQ)-induced psoriatic-like symptoms was achieved through the creation of a Irf4 -/- gene deletion mouse model and pharmacological inhibition using antisense oligonucleotides targeted for Irf4. This amelioration was accompanied by a decreased number of IL-17A-producing CD4+ T cells in the skin. The findings of this study suggest that IRF4 plays a crucial role in the promotion of inflammation and exacerbation of IMQ-induced psoriasiform dermatitis. Consequently, IRF4 targeting could be a promising therapeutic strategy.
© 2025 The Authors.
Product Citations: 274
Antisense oligonucleotides targeting IRF4 alleviate psoriasis.
In Acta Pharmaceutica Sinica. B on 1 July 2025 by Yu, Y., Wang, Y., et al.
In Frontiers in Immunology on 12 June 2025 by Jeon, J. H., Kim, S., et al.
Heterologous vaccination strategies have shown superior efficacy over homologous regimens in clinical studies, but the underlying immunological mechanisms remain incompletely understood. Using a mouse model, we investigated the immune responses induced by heterologous prime-boost vaccination with adenoviral and mRNA vaccines. Heterologous vaccination (adenoviral prime, mRNA boost) elicited higher neutralizing antibody titers and stronger CD8+ T cell responses against Delta and Omicron-BA.5 variants compared to homologous regimens. Single-cell transcriptomic analysis of injection-site tissues revealed that adenoviral priming induced minimal changes in cellular composition but established a pre-conditioned innate immune environment. This effect was further amplified upon mRNA boosting, particularly through fibroblast-driven chemokine responses that promoted immune cell recruitment. These findings suggest that adenoviral priming enhances local immune activation upon boosting, contributing to the heightened adaptive immune response observed in heterologous vaccination. This study provides mechanistic insights into the immunological effects of heterologous prime-boost strategies against SARS-CoV-2 variants.
Copyright © 2025 Jeon, Kim, Kim, Shin, Park, Chang, Kang, Kim, Park, Youk, Kim and Yeo.
-
FC/FACS
-
COVID-19
-
Immunology and Microbiology
In IMeta on 1 June 2025 by Shi, J., Mao, W., et al.
Food allergy (FA) has received increased attention in recent years. Multiple studies have highlighted the crucial role of short-chain fatty acids (SCFAs) in the development of IgE-mediated FA. Here, a case-control approach was employed to analyze SCFAs profiles in children with FA, while an ovalbumin (OVA)-sensitized mouse model was utilized to explore the underlying mechanism by which SCFAs mitigate FA. Children with food-sensitized tolerance (FST) (n = 20) or FA (n = 20), and healthy controls (HC) (n = 20) were recruited to analyze SCFAs profiles. The HC group exhibited higher SCFAs levels in fecal samples than the FST, FA, and FST + FA groups. Data from an OVA-sensitized mouse model showed that butyrate exhibited a more significant effect on reducing allergic reactions compared to other SCFAs. Compared to the negative control group, OVA-induced oxidative stress (OS) triggered excessive Notch signaling activation, which subsequently impaired both tight junctions integrity and mucosal barrier function in murine intestinal epithelial cells (IECs). Gut dysbiosis induced mucus layer erosion, thereby elevating IECs exposure to food antigens and OS, which potentiated Notch signaling activation. However, butyrate counteracted this loop by restoring microbiota structure and suppressing reactive oxygen species (ROS)/Notch cascades. Strikingly, low-dose butyrate (0.25-1 mM) protected rat small intestine crypt epithelial cells (IEC-6) by inhibiting ROS, whereas high-dose (2-5 mM) exacerbated oxidative injury and triggered activation of Notch signaling. Our study revealed the potential molecular mechanisms through which butyrate alleviates food allergy, providing a potential therapeutic strategy for its management.
© 2025 The Author(s). iMeta published by John Wiley & Sons Australia, Ltd on behalf of iMeta Science.
In Diabetes Metabolism Journal on 22 May 2025 by Song, D. G., Pak, S., et al.
Obesity is a rapidly increasing global health issue, which is associated with glucose and insulin resistance. Phosphodiesterase type 5 (PDE5) inhibitors (PDE5i) are known for their ability to enhance blood flow and vascular stability and are widely used to treat conditions such as erectile dysfunction, pulmonary hypertension, heart failure, and cancer. However, studies investigating the role of PDE5i in alleviating obesity and metabolic diseases remains unclear. Therefore, we investigated the effects of PDE5i on obesity and metabolic disorders in diet-induced obese mice and its underlying mechanisms.
PDE5i was administered to high-fat diet (HFD)-fed C57BL/6J mice for 6 to 7 weeks. Body weight and food intake were measured weekly, and baseline metabolic rates, physical activity, and glucose and insulin tolerance tests were assessed during PDE5i administration. Macrophages and T-cells in the gonadal white adipose tissue (gWAT) were analyzed by flow cytometry. Vascular stability and blood flow in gWAT were analyzed via immunostaining and in vivo live imaging. RAW264.7 cells and bone marrow-derived macrophages were used to determine immunoregulatory effects of PDE5i.
In HFD-fed mice, PDE5i administration significantly enhanced systemic insulin sensitivity and AKT phosphorylation in gWAT. PDE5i reduced the M1/M2 ratio of gWAT macrophages of obese mice. These phenomena were associated with enhanced blood flow to the gWAT. In vitro experiments revealed that PDE5i suppressed lipopolysaccharide-induced proinflammatory cytokine production and increased the mRNA expression of genes associated with M2 polarization.
PDE5i plays a role in regulating adipose tissue inflammation and thus holds promise as a therapeutic agent for metabolic enhancement.
-
Endocrinology and Physiology
-
Immunology and Microbiology
Mitochondrial fatty acid synthesis and MECR regulate CD4+ T cell function and oxidative metabolism.
In The Journal of Immunology on 1 May 2025 by Steiner, K. K., Young, A. C., et al.
Imbalanced effector and regulatory CD4+ T cell subsets drive many inflammatory diseases. These T cell subsets rely on distinct metabolic programs, modulation of which differentially affects T cell fate and function. Lipid metabolism is fundamental yet remains poorly understood across CD4+ T cell subsets. Therefore, we performed targeted in vivo CRISPR/Cas9 screens to identify lipid metabolism genes and pathways essential for T cell functions. These screens established mitochondrial fatty acid synthesis genes Mecr, Mcat, and Oxsm as key metabolic regulators. Of these, the inborn error of metabolism gene Mecr was most dynamically regulated. Mecrfl/fl; Cd4cre mice had normal naïve CD4+ and CD8+ T cell numbers, demonstrating that MECR is not essential in homeostatic conditions. However, effector and memory T cells were reduced in Mecr knockout and MECR-deficient CD4+ T cells and proliferated, differentiated, and survived less well than control T cells. Interestingly, T cells ultimately showed signs of mitochondrial stress and dysfunction in the absence of MECR. Mecr-deficient T cells also had decreased mitochondrial respiration, reduced tricarboxylic acid intermediates, and accumulated intracellular iron, which appeared to contribute to increased cell death and sensitivity to ferroptosis. Importantly, MECR-deficient T cells exhibited fitness disadvantages and were less effective at driving disease in an in vivo model of inflammatory bowel disease. Thus, MECR-mediated metabolism broadly supports CD4+ T cell proliferation and survival in vivo. These findings may also provide insight to the immunological state of MECR- and other mitochondrial fatty acid synthesis-deficient patients.
© The Author(s) 2025. Published by Oxford University Press on behalf of The American Association of Immunologists.
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
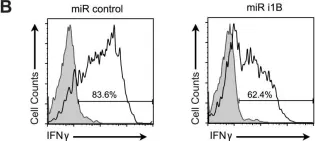
In Front Immunol on 23 July 2021 by Di Giorgio, E., Wang, L., et al.
Fig.5.E

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 3
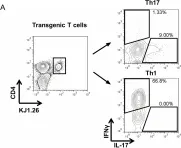
In PLoS One on 18 July 2015 by Silva, O., Crocetti, J., et al.
Fig.4.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3
In PLoS One on 21 November 2012 by Patakas, A., Benson, R. A., et al.
Fig.4.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3