Immune recognition and activation by the peptide-laden major histocompatibility complex-T cell receptor (TCR)-CD3 complex is essential for anti-tumor immunity. Tumors may escape immune surveillance by dissembling the complex. Here, we report that transferrin, which is overexpressed in patients with liver metastasis, disassociates TCR from the CD3 signaling apparatus by targeting the constant domain (CD) of T cell receptor α (TCRα), consequently suppresses T cell activation, and inhibits anti-metastatic and anti-tumor immunity. In mouse models of melanoma and lymphoma, transferrin overexpression exacerbates liver metastasis, while its knockdown, antibody, designed peptides, and CD mutation interfering with transferrin-TCRα interaction inhibit metastasis. This work reveals a novel strategy of tumor evasion of immune surveillance by blocking the coupling between TCRs and the CD3 signaling apparatus to suppress TCR activation. Given the conservation of CD and transferrin up-regulation in metastatic tumors, the strategy might be a common metastatic mechanism. Targeting transferrin-TCRα holds promise for anti-metastatic treatment.
Copyright © 2025 Ruomei Cheng et al.
Product Citations: 52
Transferrin Disassociates TCR from CD3 Signaling Apparatus to Promote Metastasis.
In Research (Washington, D.C.) on 15 January 2025 by Cheng, R., Tang, X., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
In Nature Communications on 14 November 2024 by Vijayan, A., Vogels, R., et al.
Newly approved subunit and mRNA vaccines for respiratory syncytial virus (RSV) demonstrate effectiveness in preventing severe disease, with protection exceeding 80% primarily through the generation of antibodies. An alternative vaccine platform called self-amplifying RNA (saRNA) holds promise in eliciting humoral and cellular immune responses. We evaluate the immunogenicity of a lipid nanoparticle (LNP)-formulated saRNA vaccine called SMARRT.RSV.preF, encoding a stabilized form of the RSV fusion protein, in female mice and in non-human primates (NHPs) that are either RSV-naïve or previously infected. Intramuscular vaccination with SMARRT.RSV.preF vaccine induces RSV neutralizing antibodies and cellular responses in naïve mice and NHPs. Importantly, a single dose of the vaccine in RSV pre-exposed NHPs elicits a dose-dependent anamnestic humoral immune response comparable to a subunit RSV preF vaccine. Notably, SMARRT.RSV.preF immunization significantly increases polyfunctional RSV.F specific memory CD4+ and CD8+ T-cells compared to RSV.preF protein vaccine. Twenty-four hours post immunization with SMARRT.RSV.preF, there is a dose-dependent increase in the systemic levels of inflammatory and chemotactic cytokines associated with the type I interferon response in NHPs, which is not observed with the protein vaccine. We identify a cluster of analytes including IL-15, TNFα, CCL4, and CXCL10, whose levels are significantly correlated with each other after SMARRT.RSV.preF immunization. These findings suggest saRNA vaccines have the potential to be developed as a prophylactic RSV vaccine based on innate, cellular, and humoral immune profiles they elicit.
© 2024. The Author(s).
-
Mus musculus (House mouse)
-
Genetics
-
Immunology and Microbiology
In Nature Communications on 9 November 2024 by Mao, C., Deng, F., et al.
Immune checkpoint blockade (ICB) therapy has emerged as a new therapeutic paradigm for a variety of advanced cancers, but wide clinical application is hindered by low response rate. Here we use a peptide-based, biomimetic, self-assembly strategy to generate a nanoparticle, TPM1, for binding PD-L1 on tumour cell surface. Upon binding with PD-L1, TPM1 transforms into fibrillar networks in situ to facilitate the aggregation of both bound and unbound PD-L1, thereby resulting in the blockade of the PD-1/PD-L1 pathway. Characterizations of TPM1 manifest a prolonged retention in tumour ( > 7 days) and anti-cancer effects associated with reinvigorating CD8+ T cells in multiple mice tumour models. Our results thus hint TPM1 as a potential strategy for enhancing the ICB efficacy.
© 2024. The Author(s).
-
Cancer Research
-
Immunology and Microbiology
In Communications Biology on 23 May 2024 by Kanno, T., Konno, R., et al.
Recent studies have highlighted the significance of cellular metabolism in the initiation of clonal expansion and effector differentiation of T cells. Upon exposure to antigens, naïve CD4+ T cells undergo metabolic reprogramming to meet their metabolic requirements. However, only few studies have simultaneously evaluated the changes in protein and metabolite levels during T cell differentiation. Our research seeks to fill the gap by conducting a comprehensive analysis of changes in levels of metabolites, including sugars, amino acids, intermediates of the TCA cycle, fatty acids, and lipids. By integrating metabolomics and proteomics data, we discovered that the quantity and composition of cellular lipids underwent significant changes in different effector Th cell subsets. Especially, we found that the sphingolipid biosynthesis pathway was commonly activated in Th1, Th2, Th17, and iTreg cells and that inhibition of this pathway led to the suppression of Th17 and iTreg cells differentiation. Additionally, we discovered that Th17 and iTreg cells enhance glycosphingolipid metabolism, and inhibition of this pathway also results in the suppression of Th17 and iTreg cell generation. These findings demonstrate that the utility of our combined metabolomics and proteomics analysis in furthering the understanding of metabolic transition during Th cell differentiation.
© 2024. The Author(s).
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
PAD4 controls tumor immunity via restraining the MHC class II machinery in macrophages.
In Cell Reports on 26 March 2024 by Pitter, M. R., Kryczek, I., et al.
Tumor-associated macrophages (TAMs) shape tumor immunity and therapeutic efficacy. However, it is poorly understood whether and how post-translational modifications (PTMs) intrinsically affect the phenotype and function of TAMs. Here, we reveal that peptidylarginine deiminase 4 (PAD4) exhibits the highest expression among common PTM enzymes in TAMs and negatively correlates with the clinical response to immune checkpoint blockade. Genetic and pharmacological inhibition of PAD4 in macrophages prevents tumor progression in tumor-bearing mouse models, accompanied by an increase in macrophage major histocompatibility complex (MHC) class II expression and T cell effector function. Mechanistically, PAD4 citrullinates STAT1 at arginine 121, thereby promoting the interaction between STAT1 and protein inhibitor of activated STAT1 (PIAS1), and the loss of PAD4 abolishes this interaction, ablating the inhibitory role of PIAS1 in the expression of MHC class II machinery in macrophages and enhancing T cell activation. Thus, the PAD4-STAT1-PIAS1 axis is an immune restriction mechanism in macrophages and may serve as a cancer immunotherapy target.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
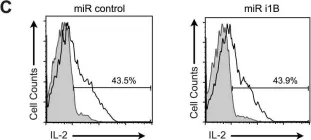
In PLoS One on 18 July 2015 by Silva, O., Crocetti, J., et al.
Fig.4.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1