After acute lesions in the central nervous system (CNS), the interaction of microglia, astrocytes, and infiltrating immune cells decides over their resolution or chronification. However, this CNS-intrinsic cross-talk is poorly characterized. Analyzing cerebrospinal fluid (CSF) samples of Multiple Sclerosis (MS) patients as well as CNS samples of female mice with experimental autoimmune encephalomyelitis (EAE), the animal model of MS, we identify microglia-derived TGFα as key factor driving recovery. Through mechanistic in vitro studies, in vivo treatment paradigms, scRNA sequencing, CRISPR-Cas9 genetic perturbation models and MRI in the EAE model, we show that together with other glial and non-glial cells, microglia secrete TGFα in a highly regulated temporospatial manner in EAE. Here, TGFα contributes to recovery by decreasing infiltrating T cells, pro-inflammatory myeloid cells, oligodendrocyte loss, demyelination, axonal damage and neuron loss even at late disease stages. In a therapeutic approach in EAE, blood-brain barrier penetrating intranasal application of TGFα attenuates pro-inflammatory signaling in astrocytes and CNS infiltrating immune cells while promoting neuronal survival and lesion resolution. Together, microglia-derived TGFα is an important mediator of glial-immune crosstalk, highlighting its therapeutic potential in resolving acute CNS inflammation.
© 2025. The Author(s).
Product Citations: 63
In Nature Communications on 19 June 2025 by Lößlein, L., Linnerbauer, M., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Inhibition of Atg7 in intestinal epithelial cells drives resistance against Citrobacter rodentium.
In Cell Death & Disease on 19 February 2025 by Cune, D., Pitasi, C. L., et al.
Autophagy, a cytoprotective mechanism in intestinal epithelial cells, plays a crucial role in maintaining intestinal homeostasis. Beyond its cell-autonomous effects, the significance of autophagy in these cells is increasingly acknowledged in the dynamic interplay between the microbiota and the immune response. In the context of colon cancer, intestinal epithelium disruption of autophagy has been identified as a critical factor influencing tumor development. This disruption modulates the composition of the gut microbiota, eliciting an anti-tumoral immune response. Here, we report that Atg7 deficiency in intestinal epithelial cells shapes the intestinal microbiota leading to an associated limitation of colitis induced by Citrobacter rodentium infection. Mice with an inducible, intestinal epithelial-cell-specific deletion of the autophagy gene, Atg7, exhibited enhanced clearance of C. rodentium, mitigated hyperplasia, and reduced pathogen-induced goblet cell loss. This protective effect is linked to a higher proportion of neutrophils and phagocytic cells in the early phase of infection. At later stages, it is associated with the downregulation of pro-inflammatory pathways and an increase in Th17 and Treg responses-immune responses known for their protective roles against C. rodentium infection, modulated by specific gut microbiota. Fecal microbiota transplantation and antibiotic treatment approaches revealed that the Atg7-deficiency-shapped microbiota, especially Gram-positive bacteria, playing a central role in driving resistance to C. rodentium infection. In summary, our findings highlight that inhibiting autophagy in intestinal epithelial cells contributes to maintaining homeostasis and preventing detrimental intestinal inflammation through microbiota-mediated colonization resistance against C. rodentium. This underscores the central role played by autophagy in shaping the microbiota in promoting immune-mediated resistance against enteropathogens.
© 2025. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Cell Biology
ERK and USP5 govern PD-1 homeostasis via deubiquitination to modulate tumor immunotherapy.
In Nature Communications on 19 May 2023 by Xiao, X., Shi, J., et al.
The programmed cell death protein 1 (PD-1) is an inhibitory receptor on T cells and plays an important role in promoting cancer immune evasion. While ubiquitin E3 ligases regulating PD-1 stability have been reported, deubiquitinases governing PD-1 homeostasis to modulate tumor immunotherapy remain unknown. Here, we identify the ubiquitin-specific protease 5 (USP5) as a bona fide deubiquitinase for PD-1. Mechanistically, USP5 interacts with PD-1, leading to deubiquitination and stabilization of PD-1. Moreover, extracellular signal-regulated kinase (ERK) phosphorylates PD-1 at Thr234 and promotes PD-1 interaction with USP5. Conditional knockout of Usp5 in T cells increases the production of effector cytokines and retards tumor growth in mice. USP5 inhibition in combination with Trametinib or anti-CTLA-4 has an additive effect on suppressing tumor growth in mice. Together, this study describes a molecular mechanism of ERK/USP5-mediated regulation of PD-1 and identifies potential combinatorial therapeutic strategies for enhancing anti-tumor efficacy.
© 2023. The Author(s).
-
FC/FACS
-
Homo sapiens (Human)
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
Chronic CD27-CD70 costimulation promotes type 1-specific polarization of effector Tregs.
In Frontiers in Immunology on 31 March 2023 by Bowakim-Anta, N., Acolty, V., et al.
Most T lymphocytes, including regulatory T cells, express the CD27 costimulatory receptor in steady state conditions. There is evidence that CD27 engagement on conventional T lymphocytes favors the development of Th1 and cytotoxic responses in mice and humans, but the impact on the regulatory lineage is unknown.
In this report, we examined the effect of constitutive CD27 engagement on both regulatory and conventional CD4+ T cells in vivo, in the absence of intentional antigenic stimulation.
Our data show that both T cell subsets polarize into type 1 Tconvs or Tregs, characterized by cell activation, cytokine production, response to IFN-γ and CXCR3-dependent migration to inflammatory sites. Transfer experiments suggest that CD27 engagement triggers Treg activation in a cell autonomous fashion.
We conclude that CD27 may regulate the development of Th1 immunity in peripheral tissues as well as the subsequent switch of the effector response into long-term memory.
Copyright © 2023 Bowakim-Anta, Acolty, Azouz, Yagita, Leo, Goriely, Oldenhove and Moser.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Impaired Treg-DC interactions contribute to autoimmunity in leukocyte adhesion deficiency type 1.
In JCI Insight on 22 December 2022 by Klaus, T., Wilson, A. S., et al.
Leukocyte adhesion deficiency type 1 (LAD-1) is a rare disease resulting from mutations in the gene encoding for the common β-chain of the β2-integrin family (CD18). The most prominent clinical symptoms are profound leukocytosis and high susceptibility to infections. Patients with LAD-1 are prone to develop autoimmune diseases, but the molecular and cellular mechanisms that result in coexisting immunodeficiency and autoimmunity are still unresolved. CD4+FOXP3+ Treg are known for their essential role in preventing autoimmunity. To understand the role of Treg in LAD-1 development and manifestation of autoimmunity, we generated mice specifically lacking CD18 on Treg (CD18Foxp3), resulting in defective LFA-1 expression. Here, we demonstrate a crucial role of LFA-1 on Treg to maintain immune homeostasis by modifying T cell-DC interactions and CD4+ T cell activation. Treg-specific CD18 deletion did not impair Treg migration into extralymphatic organs, but it resulted in shorter interactions of Treg with DC. In vivo, CD18Foxp3 mice developed spontaneous hyperplasia in lymphatic organs and diffuse inflammation of the skin and in multiple internal organs. Thus, LFA-1 on Treg is required for the maintenance of immune homeostasis.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
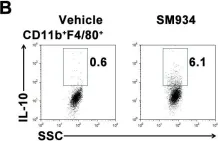
In PLoS One on 6 March 2012 by Hou, L. F., He, S. J., et al.
Fig.6.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1