The quality of T cell responses depends on the lymphocytes’ ability to undergo clonal expansion, acquire effector functions and traffic to the site of infection. Although TCR signal strength is thought to dominantly shape the T cell response, by using TCR transgenic CD4 + T cells with different pMHC binding affinity, we reveal that TCR affinity does not control Th1 effector function acquisition nor the functional output of individual effectors following mycobacterial infection. Rather, TCR affinity calibrates the rate of cell division to synchronize the distinct processes of T cell proliferation, differentiation and trafficking. By timing cell division-dependent IL-12R expression, TCR affinity controls when T cells become receptive to Th1-imprinting IL-12 signals, determining the emergence and magnitude of the Th1 effector pool. These findings reveal a distinct yet cooperative role for IL-12 and TCR signalling in Th1 differentiation and suggests that the temporal activation of clones with different TCR affinity is a major strategy to coordinate immune surveillance against persistent pathogens.
Product Citations: 36
Preprint on BioRxiv : the Preprint Server for Biology on 26 October 2020 by Bhattacharyya, N., Counoupas, C., et al.
Reciprocal regulation of Th2 and Th17 cells by PAD2-mediated citrullination.
In JCI Insight on 14 November 2019 by Sun, B., Chang, H. H., et al.
Dysregulated citrullination, a unique form of posttranslational modification catalyzed by the peptidylarginine deiminases (PADs), has been observed in several human diseases, including rheumatoid arthritis. However, the physiological roles of PADs in the immune system are still poorly understood. Here, we report that global inhibition of citrullination enhances the differentiation of type 2 helper T (Th2) cells but attenuates the differentiation of Th17 cells, thereby increasing the susceptibility to allergic airway inflammation. This effect on Th cells is due to inhibition of PAD2 but not PAD4. Mechanistically, PAD2 directly citrullinates GATA3 and RORγt, 2 key transcription factors determining the fate of differentiating Th cells. Citrullination of R330 of GATA3 weakens its DNA binding ability, whereas citrullination of 4 arginine residues of RORγt strengthens its DNA binding. Finally, PAD2-deficient mice also display altered Th2/Th17 immune response and heightened sensitivity to allergic airway inflammation. Thus, our data highlight the potential and caveat of PAD2 as a therapeutic target of Th cell-mediated diseases.
-
Mus musculus (House mouse)
In Frontiers in Immunology on 20 April 2019 by Khare, S. P., Shetty, A., et al.
SATB1 is a genome organizer protein that is expressed in a lineage specific manner in CD4+ T-cells. SATB1 plays a crucial role in expression of multiple genes throughout the thymic development and peripheral differentiation of T cells. Although SATB1 function has been subjected to intense investigation, regulation of SATB1 gene expression remains poorly understood. Analysis of RNA-seq data revealed multiple transcription start sites at the upstream regulatory region of SATB1. We further demonstrated that SATB1 gene is expressed via alternative promoters during T-helper (Th) cell differentiation. The proximal promoter "P1" is used more by the naïve and activated CD4+ T-cells whereas the middle "P2" and the distal "P3" promoters are used at a significantly higher level by polarized T-helper cells. Cytokine and TCR signaling play crucial roles toward SATB1 alternative promoter usage. Under Th2 polarization conditions, transcription factor STAT6, which operates downstream of the cytokine signaling binds to the P2 and P3 promoters. Genetic perturbation by knockout and chemical inhibition of STAT6 activation resulted in the loss of P2 and P3 promoter activity. Moreover, chemical inhibition of activation of NF-κB, a transcription factor that operates downstream of the TCR signaling, also resulted in reduced P2 and P3 promoter usage. Furthermore, usage of the P1 promoter correlated with lower SATB1 protein expression whereas P2 and P3 promoter usage correlated with higher SATB1 protein expression. Thus, the promoter switch might play a crucial role in fine-tuning of SATB1 protein expression in a cell type specific manner.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Journal of Interferon & Cytokine Research on 1 April 2019 by Skrombolas, D., Sullivan, M., et al.
Interleukin-12 (IL-12) is a pleiotropic cytokine that has profound effects on many aspects of cell-mediated responses and can enhance antitumor responses in experimental models. IL-12 has been tested clinically, however, side-effects have limited its use. We are developing an attenuated form of IL-12 whose biological activity could be restricted to sites of tumors by taking advantage of overexpressed tumor proteases that can activate the cytokine. We constructed a panel of fusion proteins (FPs) consisting of IL-12 joined to a specific inhibitor connected by a protease cleavage sequence (cs). We first identified a panel of single-chain Fragment variable (scFv) that bind to 3 independent epitopes on IL-12 and then incorporated them into separate IL-12 FPs containing either a matrix metalloproteinase (MMP) cs or a scrambled (scram) control cs. The intact IL-12 FPs showed attenuation in IL-12 activity compared to free IL-12 in 2 separate in vitro functional assays; proliferation of CTLL-2 and interferon-gamma (IFN-γ) induction by spleen cells. Furthermore, the FP containing the MMPcs showed an increase in biological activity of IL-12 in vitro when cleaved by MMP9. This FP strategy could be applied to other immunomodulators and potentially reduce unwanted side-effects observed with systemic delivery thus improving cytokine immunotherapy strategies.
In The Journal of Immunology on 1 November 2018 by Lim, H. X., Jung, H. J., et al.
In addition to essential roles in protein synthesis, lysyl-tRNA synthetase (KRS) is secreted to trigger a proinflammatory function that induces macrophage activation and TNF-α secretion. KRS has been associated with autoimmune diseases such as polymyositis and dermatomyositis. In this study, we investigated the immunomodulatory effects of KRS on bone marrow-derived dendritic cells (DCs) of C57BL/6 mice and subsequent polarization of Th cells and analyzed the underlying mechanisms. KRS-treated DCs increased the expression of cell surface molecules and proinflammatory cytokines associated with DC maturation and activation. Especially, KRS treatment significantly increased production of IL-12, a Th1-polarizing cytokine, in DCs. KRS triggered the nuclear translocation of the NF-κB p65 subunit along with the degradation of IκB proteins and the phosphorylation of MAPKs in DCs. Additionally, JNK, p38, and ERK inhibitors markedly recovered the degradation of IκB proteins, suggesting the involvement of MAPKs as the upstream regulators of NF-κB in the KRS-induced DC maturation and activation. Importantly, KRS-treated DCs strongly increased the differentiation of Th1 cells when cocultured with CD4+ T cells. The addition of anti-IL-12-neutralizing Ab abolished the secretion of IFN-γ in the coculture, indicating that KRS induces Th1 cell response via DC-derived IL-12. Moreover, KRS enhanced the OVA-specific Th1 cell polarization in vivo following the adoptive transfer of OVA-pulsed DCs. Taken together, these results indicated that KRS effectively induced the maturation and activation of DCs through MAPKs/NF-κB-signaling pathways and favored DC-mediated Th1 cell response.
Copyright © 2018 by The American Association of Immunologists, Inc.
-
Genetics
-
Immunology and Microbiology
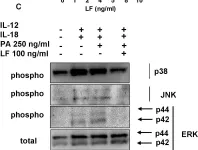
In PLoS Pathog on 1 January 2012 by Klezovich-Bénard, M., Corre, J. P., et al.
Fig.4.C

-
WB
-
Collected and cropped from PLoS Pathog by CiteAb, provided under a CC-BY license
Image 1 of 1