The clinical potential of current chimeric antigen receptor-engineered T (CAR-T) cell therapy is hampered by its autologous nature that poses considerable challenges in manufacturing, costs and patient selection. This spurs demand for off-the-shelf therapies. Here we introduce an ex vivo feeder-free culture method to differentiate gene-engineered hematopoietic stem and progenitor (HSP) cells into allogeneic invariant natural killer T (AlloNKT) cells and their CAR-armed derivatives (AlloCAR-NKT cells). We include detailed information on lentivirus generation and titration, as well as the five stages of ex vivo culture required to generate AlloCAR-NKT cells, including HSP cell engineering, HSP cell expansion, NKT cell differentiation, NKT cell deep differentiation and NKT cell expansion. In addition, we describe procedures for evaluating the pharmacology, antitumor efficacy and mechanism of action of AlloCAR-NKT cells. It takes ~2 weeks to generate and titrate lentiviruses and ~6 weeks to generate mature AlloCAR-NKT cells. Competence with human stem cell and T cell culture, gene engineering and flow cytometry is required for optimal results.
© 2025. Springer Nature Limited.
Product Citations: 65
In Nature Protocols on 1 May 2025 by Li, Y. R., Zhou, K., et al.
-
Cancer Research
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 20 April 2025 by Ormhøj, M., Munk, K. K., et al.
Adoptive cell therapy (ACT) has shown promising results in cancer treatment, however, achieving effective ex vivo expansion of potent, functionally active, and cytotoxic T cells remains challenging. To overcome this, we loaded the engineered cytokine Neoleukin-2/15 (Neo2/15) on our recently established artificial antigen-presenting scaffolds (Ag-scaffolds) to expand antigen-specific T cells. Neo2/15 selectively binds to IL-2Rβ/γ receptors, enhancing CD8 + T cell proliferation while limiting regulatory T cell expansion. Our study assessed the efficacy of Neo2/15-loaded Ag-scaffolds (Ag-Neo2/15 scaffolds) in expanding antigen-specific T cells from peripheral blood mononuclear cells (PBMCs) of healthy donors. We optimized Ag-scaffold configurations by varying the number of Neo2/15 molecules loaded on Ag-scaffolds and evaluated their impact on T-cell expansion and functionality. We showed that Ag-Neo2/15 scaffolds promoted significant T-cell expansion, with a comparable frequency of antigen-specific CD8 + T cells compared to IL-2/IL-21-loaded Ag-scaffolds (Ag-IL2/21 scaffolds). The CD8 + T cells expanded with Ag-Neo2/15 scaffolds exhibited potent TNFα and IFNγ production and expressed high levels of α4β7 integrin, a homing molecule which is important for directing T cells to specific tissues, potentially enhancing their therapeutic potential. T cells expanded with Ag-Neo2/15 scaffolds had superior and durable cytotoxicity against tumor target cells compared to T cells expanded with Ag-IL2/21 scaffolds. These findings were further supported by our single-cell analysis revealing that T cells expanded with Ag-Neo2/15 scaffolds had higher cytotoxic scores and lower dysfunctionality scores compared to T cells expanded with Ag-IL2/21 scaffolds. The single-cell analysis also indicated increased expression of genes linked to cell division and enhanced proliferative capacity in Ag-Neo2/15 expanded T cells. Furthermore, TCR clonality analysis demonstrated that Ag-Neo2/15 scaffolds promoted the expansion of functionally superior T-cell clones. The top clones of CD8 + T cells expanded with Ag-Neo2/15 scaffolds exhibited a favorable phenotype, essential for effective antigen recognition and sustained T-cell mediated cytotoxicity. Our findings suggest that Ag-Neo2/15 scaffolds represent an advancement in ACT by producing high-quality, functional antigen-specific T cells. This method has the potential to improve clinical outcomes in cancer therapy by generating large numbers of highly functional T cells, thereby optimizing the balance between cytotoxicity and proliferation capacity with less exhausted T-cells in expansion protocols.
-
Immunology and Microbiology
In The Journal of Clinical Investigation on 13 August 2024 by Lledó-Delgado, A., Preston-Hurlburt, P., et al.
BACKGROUNDTeplizumab, a non-FcR-binding anti-CD3 mAb, is approved to delay progression of type 1 diabetes (T1D) in at-risk patients. Previous investigations described the immediate effects of the 14-day treatment, but longer-term effects of the drug remain unknown.METHODSWith an extended analysis of study participants, we found that 36% were undiagnosed or remained free of clinical diabetes after 5 years, suggesting operational tolerance. Using single-cell RNA sequencing, we compared the phenotypes, transcriptome, and repertoire of peripheral blood CD8+ T cells including autoreactive T cells from study participants before and after teplizumab and features of responders and non-responders.RESULTSAt 3 months, there were transcriptional signatures of cell activation in CD4+ and CD8+ T cells including signaling that was reversed at 18 months. At that time, there was reduced expression of genes in T cell receptor and activation pathways in clinical responders. In CD8+ T cells, we found increased expression of genes associated with exhaustion and immune regulation with teplizumab treatment. These transcriptional features were further confirmed in an independent cohort. Pseudotime analysis showed differentiation of CD8+ exhausted and memory cells with teplizumab treatment. IL7R expression was reduced, and patients with lower expression of CD127 had longer diabetes-free intervals. In addition, the frequency of autoantigen-reactive CD8+ T cells, which expanded in the placebo group over 18 months, did not increase in the teplizumab group.CONCLUSIONThese findings indicate that teplizumab promotes operational tolerance in T1D, involving activation followed by exhaustion and regulation, and prevents expansion of autoreactive T cells.TRIAL REGISTRATIONClinicalTrials.gov NCT01030861.FUNDINGNational Institute of Diabetes and Digestive and Kidney Diseases/NIH, Juvenile Diabetes Research Foundation.
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Frontiers in Immunology on 14 May 2024 by Kim, E. S., Islam, J., et al.
Tuberculous pleural effusion (TPE) stands as one of the primary forms of extrapulmonary tuberculosis (TB) and frequently manifests in regions with a high prevalence of TB, consequently being a notable cause of pleural effusion in such areas. However, the differentiation between TPE and parapneumonic pleural effusion (PPE) presents diagnostic complexities. This study aimed to evaluate the potential of myeloid-derived suppressor cells (MDSCs) in the pleural fluid as a potential diagnostic marker for distinguishing between TPE and PPE.
Adult patients, aged 18 years or older, who presented to the emergency room of a tertiary referral hospital and received a first-time diagnosis of pleural effusion, were prospectively enrolled in the study. Various immune cell populations, including T cells, B cells, natural killer (NK) cells, and MDSCs, were analyzed in both pleural fluid and peripheral blood samples.
In pleural fluid, the frequency of lymphocytes, including T, B, and NK cells, was notably higher in TPE compared to PPE. Conversely, the frequency of polymorphonuclear (PMN)-MDSCs was significantly higher in PPE. Notably, compared to traditional markers such as the neutrophil-to-lymphocyte ratio and adenosine deaminase level, the frequency of PMN-MDSCs emerged as a more effective discriminator between PPE and TPE. PMN-MDSCs demonstrated superior positive and negative predictive values and exhibited a higher area under the curve in the receiver operating characteristic curve analysis. PMN-MDSCs in pleural effusion increased the levels of reactive oxygen species and suppressed the production of interferon-gamma from T cells following nonspecific stimulation. These findings suggest that MDSC-mediated immune suppression may contribute to the pathology of both TPE and PPE.
The frequency of PMN-MDSCs in pleural fluid is a clinically useful indicator for distinguishing between TPE and PPE.
Copyright © 2024 Kim, Islam, Lee, Seong, Youn, Kwon, Kim and Lee.
-
Homo sapiens (Human)
-
Cardiovascular biology
-
Immunology and Microbiology
In Cell Reports Medicine on 20 February 2024 by Prinz, L. F., Riet, T., et al.
Chimeric antigen receptor T cell (CAR T) therapy is a potent treatment for relapsed/refractory (r/r) B cell lymphomas but provides lasting remissions in only ∼40% of patients and is associated with serious adverse events. We identify an upregulation of CD80 and/or CD86 in tumor tissue of (r/r) diffuse large B cell lymphoma (DLBCL) patients treated with tisagenlecleucel. This finding leads to the development of the CAR/CCR (chimeric checkpoint receptor) design, which consists of a CD19-specific first-generation CAR co-expressed with a recombinant CTLA-4-linked receptor with a 4-1BB co-stimulatory domain. CAR/CCR T cells demonstrate superior efficacy in xenograft mouse models compared with CAR T cells, superior long-term activity, and superior selectivity in in vitro assays with non-malignant CD19+ cells. In addition, immunocompetent mice show an intact CD80-CD19+ B cell population after CAR/CCR T cell treatment. The results reveal the CAR/CCR design as a promising strategy for further translational study.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
ELISA
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Nat Commun on 22 February 2021 by Park, J. J., Thi, E. P., et al.
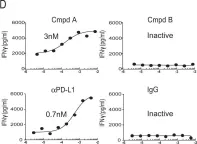
Fig.1.D

-
ELISA
-
Cricetulus griseus (Chinese hamster)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 2
In Nat Commun on 22 February 2021 by Park, J. J., Thi, E. P., et al.
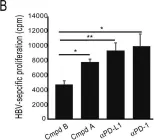
Fig.4.B

-
ELISA
-
Cricetulus griseus (Chinese hamster)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 2