Hypertension is a primary risk factor for the development of cardiovascular disease. Mechanisms controlling blood pressure (BP) in men and women are still being investigated; however, there is increasing evidence supporting a role for the innate immune system. Specifically, Toll-like receptors (TLRs), and TLR4 in particular, have been implicated in the development of hypertension in male spontaneously hypertensive rats (SHR). Despite established sex differences in BP control and inflammatory markers in hypertensive males and females, little is known regarding the role of TLR4 in hypertension in females. Our hypotheses were that male SHR have greater TLR4 expression compared with females, and that sex differences in TLR4 contribute to sex differences in BP and the T cell profile. To test these hypotheses, initial studies measured renal TLR4 protein expression in 13-wk-old male and female SHR. Additional SHR were implanted with telemetry devices and randomized to treatment with either IgG or TLR4 neutralizing antibodies. Untreated control male SHR have greater TLR4 protein expression in the kidney compared with females. However, treatment with TLR4 neutralizing antibody for 2 wk did not significantly alter BP in either male or female SHR. Interestingly, neutralization of TLR4 increased renal CD3+ T cells in female SHR, with no alteration in CD4+ T cells or CD8+ T cells in either sex. Taken together, our data indicate that although male SHR have greater renal TLR4 expression than females, TLR4 does not contribute to the higher BP and more proinflammatory renal T cell profile in males versus females.
Product Citations: 20
In American Journal of Physiology - Regulatory, Integrative and Comparative Physiology on 1 April 2022 by Belanger, K. M., Mohamed, R., et al.
-
Cardiovascular biology
-
Endocrinology and Physiology
-
Immunology and Microbiology
In Journal of Neuroinflammation on 19 October 2021 by Choi, J. H., Oh, J., et al.
Lysophosphatidic acid receptors (LPARs) are G-protein-coupled receptors involved in many physiological functions in the central nervous system. However, the role of the LPARs in multiple sclerosis (MS) has not been clearly defined yet.
Here, we investigated the roles of LPARs in myelin oligodendrocyte glycoprotein peptides-induced experimental autoimmune encephalomyelitis (EAE), an animal model of MS.
Pre-inhibition with LPAR1-3 antagonist Ki16425 deteriorated motor disability of EAElow. Specifically, LPAR1-3 antagonist (intraperitoneal) deteriorated symptoms of EAElow associated with increased demyelination, chemokine expression, cellular infiltration, and immune cell activation (microglia and macrophage) in spinal cords of mice compared to the sham group. This LPAR1-3 antagonist also increased the infiltration of CD4+/IFN-γ+ (Th1) and CD4+/IL-17+ (Th17) cells into spinal cords of EAElow mice along with upregulated mRNA expression of IFN-γ and IL-17 and impaired blood-brain barrier (BBB) in the spinal cord. The underlying mechanism for negative effects of LPAR1-3 antagonist was associated with the overproduction of reactive oxygen species (ROS)-generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) 2 and NOX3. Interestingly, LPAR1/2 agonist 1-oleoyl-LPA (LPA 18:1) (intraperitoneal) ameliorated symptoms of EAEhigh and improved representative pathological features of spinal cords of EAEhigh mice.
Our findings strongly suggest that some agents that can stimulate LPARs might have potential therapeutic implications for autoimmune demyelinating diseases such as MS.
© 2021. The Author(s).
-
Immunology and Microbiology
In Molecular Medicine on 3 September 2021 by Zhu, L., Xu, F., et al.
N-Acetylcysteine (NAC) demonstrates applications in the prevention of exacerbation of chronic obstructive pulmonary disease (COPD). COPD is often characterized by fibrosis of the small airways. This study aims at investigating the physiological mechanisms by which NAC might mediate the pulmonary fibrosis in COPD.
A total of 10 non-smokers without COPD and 10 smokers with COPD were recruited in this study, and COPD rat models were established. Cigarette smoke extract (CSE) cell models were constructed. The gain- or loss-of-function experiments were adopted to determine the expression of VWF and the extent of p38 MAPK phosphorylation, levels of interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and immunoglobulins (IgG, IgM and IgA) in the serum of COPD rats and supernatant of alveolar epithelial cells and to detect cell invasion and migration and the ratio of CD3+, CD4+, CD8+ and CD4+/CD8+T lymphocytes.
Expression of VWF and the extent of p38 MAPK phosphorylation were increased in COPD. NAC inhibited p38 MAPK phosphorylation by reducing the VWF expression. NAC could inhibit cell migration and invasion, elevate E-cadherin expression, the ratio of CD3+, CD4+, CD8+ and CD4+/CD8+T lymphocytes, and levels of IgG, IgA, and IgM, and reduce N-cadherin expression and levels of IL-6 and TNF-α in CSE cells and serum of COPD rats. NAC promoted immune response and suppressed epithelial-mesenchymal transformation (EMT) to relieve COPD-induced pulmonary fibrosis in vitro and in vivo by inhibiting the VWF/p38 MAPK axis.
Collectively, NAC could ameliorate COPD-induced pulmonary fibrosis by promoting immune response and inhibiting EMT process via the VWF/p38 MAPK axis, therefore providing us with a potential therapeutic target for treating COPD.
© 2021. The Author(s).
-
FC/FACS
-
Rattus norvegicus (Rat)
-
Biochemistry and Molecular biology
-
Cardiovascular biology
-
Immunology and Microbiology
In Antioxidants (Basel, Switzerland) on 16 October 2020 by Tanner, M. R., Huq, R., et al.
Reactive oxygen species have been involved in the pathogenesis of rheumatoid arthritis (RA). Our goal was to determine the effects of selectively scavenging superoxide (O2•-) and hydroxyl radicals with antioxidant nanoparticles, called poly(ethylene glycol)-functionalized hydrophilic carbon clusters (PEG-HCCs), on the pathogenic functions of fibroblast-like synoviocytes (FLS) from patients with rheumatoid arthritis (RA) and on the progression of an animal model of RA. We used human FLS from patients with RA to determine PEG-HCC internalization and effects on FLS cytotoxicity, invasiveness, proliferation, and production of proteases. We used the pristane-induced arthritis (PIA) rat model of RA to assess the benefits of PEG-HCCs on reducing disease severity. PEG-HCCs were internalized by RA-FLS, reduced their intracellular O2•-, and reduced multiple measures of their pathogenicity in vitro, including proliferation and invasion. In PIA, PEG-HCCs caused a 65% reduction in disease severity, as measured by a standardized scoring system of paw inflammation and caused a significant reduction in bone and tissue damage, and circulating rheumatoid factor. PEG-HCCs did not induce lymphopenia during PIA. Our study demonstrated a role for O2•- and hydroxyl radicals in the pathogenesis of a rat model of RA and showed efficacy of PEG-HCCs in treating a rat model of RA.
-
FC/FACS
-
Rattus norvegicus (Rat)
In Journal of Toxicologic Pathology on 1 July 2019 by Ogawa, B., Nakanishi, Y., et al.
When conducting histopathological evaluation of lymphoid tissues, it is necessary to know the variability and strain differences in histological features of different sites of lymphoid tissues. To investigate in detail the variability of lymphoid tissues and strain differences of control rats as well as those of immune reactivity and sensitivity to immunosuppression, we performed a histopathological analysis of various lymphoid tissues in conjunction with the evaluation of immune function in a T cell-dependent antibody response (TDAR) assay with cyclophosphamide (CP) in Sprague Dawley (SD) and F344 rats. Six-week-old male SD and F344 rats were orally treated with CP at 0 (control) or 4 mg/kg/day for 28 days; keyhole limpet hemocyanin (KLH) was introduced intravenously on Days 14 and 23, and the serum concentrations of anti-KLH antibodies were measured. HE staining and immunohistochemistry for T-cell (CD3) and B-cell (CD45RA) markers were performed using tissues from the spleen, thymus, and various lymph nodes. In CP-treated rats of both strains, decreased concentrations of anti-KLH antibodies were observed. Histopathological analysis revealed decreased lymphocytes mainly in the B-cell area, and these changes induced by CP treatment were more prominent in the F344 rats than in the SD rats. The present study also demonstrated that some of the lymphoid tissues of the control F344 rats were less developed than those of the control SD rats, suggesting that F344 rats might be easily affected by CP-induced immunosuppression. This information concerning rat strain differences in lymphoid tissues will be useful in histopathological evaluation for drug-induced immunotoxicity.
-
Immunology and Microbiology
-
Pathology
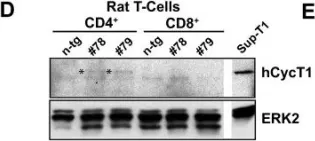
In Retrovirology on 13 January 2009 by Michel, N., Goffinet, C., et al.
Fig.1.D

-
WB
-
Collected and cropped from Retrovirology by CiteAb, provided under a CC-BY license
Image 1 of 1