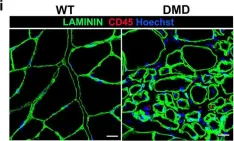
Duchenne muscular dystrophy (DMD) is a fatal muscle-wasting disorder caused by mutations in the Dystrophin gene and for which there is currently no cure. To bridge the gap between preclinical and therapeutic evaluation studies, we have generated a rat model for DMD that carries an exon 52 deletion (R-DMDdel52) causing a complete lack of dystrophin protein. Here we show that R-DMDdel52 animals recapitulated human DMD pathophysiological trajectory more faithfully than the mdx mouse model. We report that R-DMDdel52 rats displayed progressive and severe skeletal muscle loss associated with fibrotic deposition, fat infiltration and fibre type switch. Early fibrosis was also apparent in the cardiac muscle. These histological modifications led to severe muscle, respiratory and cardiac functional impairments leading to premature death around 1 year. Moreover, DMD muscle exhibited systemic inflammation with a mixed M1/M2 phenotype. A comparative single cell RNAseq analysis of the diaphragm muscle was performed, revealing cellular populations alteration and molecular modifications in all muscle cell types. We show that DMD fibroadipogenic progenitors produced elevated levels of cartilage oligomeric matrix protein, a glycoprotein responsible for modulating homeostasis of extracellular matrix, and whose increased concentration correlated with muscle fibrosis both in R-DMDdel52 rats and human patients. Fibrosis is a component of tissue remodelling impacting the whole musculature of DMD patients, at the tissue level but most importantly at the functional level. We therefore propose that this specific biomarker can optimize the prognostic monitoring of functional improvement of patients included in clinical trials.
© 2022. The Author(s).
Product Citations: 20
In Acta Neuropathologica Communications on 25 April 2022 by Taglietti, V., Kefi, K., et al.
-
IHC
-
Rattus norvegicus (Rat)
In Scientific Reports on 30 November 2021 by Sousa, G. C., Fernandes, M. V., et al.
Acute ischemic stroke is associated with pulmonary complications, and often dexmedetomidine and propofol are used to decrease cerebral metabolic rate. However, it is unknown the immunomodulatory actions of dexmedetomidine and propofol on brain and lungs during acute ischemic stroke. The effects of dexmedetomidine and propofol were compared on perilesional brain tissue and lung damage after acute ischemic stroke in rats. Further, the mean amount of both sedatives was directly evaluated on alveolar macrophages and lung endothelial cells primarily extracted 24-h after acute ischemic stroke. In twenty-five Wistar rats, ischemic stroke was induced and after 24-h treated with sodium thiopental (STROKE), dexmedetomidine and propofol. Dexmedetomidine, compared to STROKE, reduced diffuse alveolar damage score [median(interquartile range); 12(7.8-15.3) vs. 19.5(18-24), p = 0.007)], bronchoconstriction index [2.28(2.08-2.36) vs. 2.64(2.53-2.77), p = 0.006], and TNF-α expression (p = 0.0003), while propofol increased VCAM-1 expression compared to STROKE (p = 0.0004). In perilesional brain tissue, dexmedetomidine, compared to STROKE, decreased TNF-α (p = 0.010), while propofol increased VCAM-1 compared to STROKE (p = 0.024). In alveolar macrophages and endothelial cells, dexmedetomidine decreased IL-6 and IL-1β compared to STROKE (p = 0.002, and p = 0.040, respectively), and reduced IL-1β compared to propofol (p = 0.014). Dexmedetomidine, but not propofol, induced brain and lung protection in experimental acute ischemic stroke.
© 2021. The Author(s).
-
Rattus norvegicus (Rat)
-
Cardiovascular biology
In Journal of Tissue Engineering and Regenerative Medicine on 1 October 2020 by Harper, S., Hoff, M., et al.
Liver transplantation is the only life-saving treatment for end-stage liver failure but is limited by the organ shortage and consequences of immunosuppression. Repopulation of decellularised scaffolds with recipient cells provides a theoretical solution, allowing reliable and timely organ sourcing without the need for immunosuppression. Recellularisation of the vasculature of decellularised liver scaffolds was investigated as an essential prerequisite to the survival of other parenchymal components. Liver decellularisation was carried out by portal vein perfusion using a detergent-based solution. Decellularised scaffolds were placed in a sterile perfusion apparatus consisting of a sealed organ chamber, functioning at 37°C in normal atmospheric conditions. The scaffold was perfused via portal vein with culture medium. A total of 107 primary cultured bone marrow stem cells, selected by plastic adherence, were infused into the scaffold, after which repopulated scaffolds were perfused for up to 30 days. The cultured stem cells were assessed for key marker expression using fluorescence-activated cell sorting (FACS), and recellularised scaffolds were analysed by light, electron and immunofluorescence microscopy. Stem cells were engrafted in portal, sinusoidal and hepatic vein compartments, with cell alignment reminiscent of endothelium. Cell surface marker expression altered following engraftment, from haematopoietic to endothelial phenotype, and engrafted cells expressed sinusoidal endothelial endocytic receptors (mannose, Fc and stabilin receptors). These results represent one step towards complete recellularisation of the liver vasculature and progress towards the objective of generating transplantable neo-organs.
© 2020 John Wiley & Sons, Ltd.
-
Cardiovascular biology
-
Stem Cells and Developmental Biology
Resuscitation From Hemorrhagic Shock With Fresh and Stored Blood and Polymerized Hemoglobin.
In Shock (Augusta, Ga.) on 1 October 2020 by Williams, A. T., Lucas, A., et al.
Hemoglobin (Hb)-based oxygen carriers (HBOCs) have been proposed as alternatives to blood for decades. Previous studies demonstrated that large molecular diameter HBOCs based on polymerized bovine Hb (PolybHb) attenuate Hb side-effects and toxicity. The objective of this study was to test the safety and efficacy of tense state PolybHb after long-term storage.
PolybHb was subjected to diafiltration to remove low molecular weight (< 500 kDa) species and stored for 2 years. PolybHb was studied in parallel with blood, collected from rats and stored leukodepleted under blood bank conditions for 3 weeks. Rats were hemorrhaged and resuscitated to 90% of the blood pressure before the hemorrhage with fresh blood, stored blood, fresh PolybHb, or 2-year-stored PolybHb. Hemorrhagic shock impaired oxygen delivery and cardiac function. Resuscitation restored blood pressure and cardiac function, but stored blood required a significantly larger transfusion volume to recover from shock compared with fresh blood and PolybHb (fresh and stored). Stored blood transfusion elevated markers of organ damage compared with all other groups.
These studies indicate that large molecular diameter PolybHb is as efficacious as fresh blood in restoring cardiac function and confirm the lack of degradation of PolybHb's safety or efficacy during long-term storage.
-
FC/FACS
-
Rattus norvegicus (Rat)
-
Cardiovascular biology
Knockout of reactive astrocyte activating factors slows disease progression in an ALS mouse model.
In Nature Communications on 27 July 2020 by Guttenplan, K. A., Weigel, M. K., et al.
Reactive astrocytes have been implicated in the pathogenesis of neurodegenerative diseases, including a non-cell autonomous effect on motor neuron survival in ALS. We previously defined a mechanism by which microglia release three factors, IL-1α, TNFα, and C1q, to induce neurotoxic astrocytes. Here we report that knocking out these three factors markedly extends survival in the SOD1G93A ALS mouse model, providing evidence for gliosis as a potential ALS therapeutic target.
-
Neuroscience
In Acta Neuropathol Commun on 25 April 2022 by Taglietti, V., Kefi, K., et al.
Fig.6.I

-
IHC
-
Rattus norvegicus (Rat)
Collected and cropped from Acta Neuropathol Commun by CiteAb, provided under a CC-BY license
Image 1 of 1