Spark-discharged anodic oxidation coating on commercially pure titanium (SAc.p.Ti) has been shown to promote bone conduction and bone matrix mineralization during new bone formation. This study hypothesized that the combination of SAc.p.Ti with dental pulp stem cells (DPSCs) would enhance new bone formation. The objective was to evaluate the effect of this combination in a rat bone defect model.
DPSCs were isolated from Sprague-Dawley (SD) rat incisors and cultured. Calvarial bone defects were created in SD rats, followed by transplantation of commercially pure titanium (c.p.Ti), SAc.p.Ti, or SAc.p.Ti combined with DPSCs. Bone formation was assessed using micro-computed tomography (micro-CT). Toluidine blue O staining was employed to evaluate bone-implant contact and the newly formed bone area. Hematoxylin-eosin (HE) staining was performed to identify osteoblast-like cells.
Micro-CT analysis revealed hard tissue formation on the surface of SAc.p.Ti. Toluidine blue O staining showed significantly greater bone-implant contact and newly formed bone area in the SAc.p.Ti/DPSC group compared to the c.p.Ti and SAc.p.Ti groups. HE staining confirmed the presence of osteoblast-like cells at the defect margins, with evidence of new bone formation on the surface of SAc.p.Ti and in the SAc.p.Ti/DPSC groups.
The combination of SAc.p.Ti and DPSCs presents a promising strategy for promoting new bone formation in rat calvarial defect model.
Product Citations: 24
In Journal of Prosthodontic Research on 11 February 2025 by Kobayashi, T., Hata, M., et al.
-
Rattus norvegicus (Rat)
-
Stem Cells and Developmental Biology
TRPM2 Is Not Required for T-Cell Activation and Differentiation.
In Frontiers in Immunology on 1 February 2022 by Lory, N. C., Nawrocki, M., et al.
Antigen recognition by the T-cell receptor induces a cytosolic Ca2+ signal that is crucial for T-cell function. The Ca2+ channel TRPM2 (transient receptor potential cation channel subfamily M member 2) has been shown to facilitate influx of extracellular Ca2+ through the plasma membrane of T cells. Therefore, it was suggested that TRPM2 is involved in T-cell activation and differentiation. However, these results are largely derived from in vitro studies using T-cell lines and non-physiologic means of TRPM2 activation. Thus, the relevance of TRPM2-mediated Ca2+ signaling in T cells remains unclear. Here, we use TRPM2-deficient mice to investigate the function of TRPM2 in T-cell activation and differentiation. In response to TCR stimulation in vitro, Trpm2-/- and WT CD4+ and CD8+ T cells similarly upregulated the early activation markers NUR77, IRF4, and CD69. We also observed regular proliferation of Trpm2-/- CD8+ T cells and unimpaired differentiation of CD4+ T cells into Th1, Th17, and Treg cells under specific polarizing conditions. In vivo, Trpm2-/- and WT CD8+ T cells showed equal specific responses to Listeria monocytogenes after infection of WT and Trpm2-/- mice and after transfer of WT and Trpm2-/- CD8+ T cells into infected recipients. CD4+ T-cell responses were investigated in the model of anti-CD3 mAb-induced intestinal inflammation, which allows analysis of Th1, Th17, Treg, and Tr1-cell differentiation. Here again, we detected similar responses of WT and Trpm2-/- CD4+ T cells. In conclusion, our results argue against a major function of TRPM2 in T-cell activation and differentiation.
Copyright © 2022 Lory, Nawrocki, Corazza, Schmid, Schumacher, Bedke, Menzel, Koch-Nolte, Guse, Huber and Mittrücker.
-
Immunology and Microbiology
In Journal of Prosthodontic Research on 15 October 2021 by Aoyagi, A., Hata, M., et al.
Purpose Implants made of anodized-hydrothermally treated commercially pure titanium with a nanotopographic surface structure (SA-treated c.p.Ti) may advantageously promote contact osteogenesis during the early stages of healing. We hypothesized that utilizing SA-treated c.p.Ti with dental pulp stem cells (DPSCs) might improve osteoconduction during the process of osseointegration. This in vitro study investigated the effect of initial adhesion of DPSCs to SA-treated c.p.Ti compared with conventional c.p.Ti and anodic oxide (AO) c.p.Ti.Methods DPSCs were obtained from the mandibular incisors of Sprague-Dawley rats and cultured without osteogenic induction medium on c.p.Ti, AO c.p.Ti, and SA-treated c.p.Ti disks for up to 14 days. The morphology, proliferation, and differentiation of DPSCs were assessed by scanning electron microscopy, an MTT assay, and Alizarin Red S staining, respectively. A real-time quantitative polymerase chain reaction was used to quantify the mRNA expression of osteocalcin, osteopontin, and bone sialoprotein.Results On all disks, the DPSCs appeared flattened with the formation of extensions over time. The filopodium-like extensions were closely bound to the SA-treated c.p.Ti surface. The proliferation of DPSCs was not significantly different among the c.p.Ti treatments. However, DPSCs on SA-treated c.p.Ti showed the greatest mRNA levels of osteopontin, osteocalcin, and bone sialoprotein, as well as increased Alizarin Red S staining.Conclusions The results of the present in vitro study demonstrate that the surface properties of SA-treated c.p.Ti disks enhance osteogenic differentiation of DPSCs and may facilitate mineralized matrix formation on SA-treated c.p.Ti implant surfaces, which can enhance early bone regeneration.
-
Rattus norvegicus (Rat)
-
Stem Cells and Developmental Biology
In The Journal of Experimental Medicine on 5 April 2021 by Kiuchi, M., Onodera, A., et al.
Different dynamics of gene expression are observed during cell differentiation. In T cells, genes that are turned on early or turned off and stay off have been thoroughly studied. However, genes that are initially turned off but then turned on again after stimulation has ceased have not been defined; they are obviously important, especially in the context of acute versus chronic inflammation. Using the Th1/Th2 differentiation paradigm, we found that the Cxxc1 subunit of the Trithorax complex directs transcription of genes initially down-regulated by TCR stimulation but up-regulated again in a later phase. The late up-regulation of these genes was impaired either by prolonged TCR stimulation or Cxxc1 deficiency, which led to decreased expression of Trib3 and Klf2 in Th1 and Th2 cells, respectively. Loss of Cxxc1 resulted in enhanced pathogenicity in allergic airway inflammation in vivo. Thus, Cxxc1 plays essential roles in the establishment of a proper CD4+ T cell immune system via epigenetic control of a specific set of genes.
© 2021 Kiuchi et al.
-
Genetics
-
Immunology and Microbiology
In International Journal of Molecular Medicine on 1 August 2020 by Zhao, K., Liu, J., et al.
Diabetic retinopathy (DR) is one of the most prevalent microvascular complications of diabetes, and a common cause of blindness in working‑age individuals. Mesenchymal stem cell (MSC) transplantation has been considered a promising intervention therapy for DR, wherein the differentiation of MSCs into nerve cells plays an essential role. However, research into the role of MSCs in DR treatment remains incomplete, and the mechanisms of retinal repair at the molecular level have yet to be clarified. In the present study, all‑trans retinoic acid (ATRA) was used to promote the proliferation of rat umbilical cord (UC)‑derived MSCs and their differentiation into nerve cells. Furthermore, the effects and mechanisms of UC‑MSCs with or without ATRA treatment were investigated in rats subjected to streptozocin (STZ)‑induced DR. The results demonstrated that the transplantation of UC‑MSCs treated with or without ATRA attenuated DR in rats, and alleviated retinal tissue damage and apoptosis. In addition, the transplantation of UC‑MSCs treated with or without ATRA attenuated angiogenesis and inflammation in the retina by regulating the levels of relevant cytokines. UC‑MSCs treated with ATRA exerted a more prominent therapeutic effect than the untreated UC‑MSCs. On the whole, these findings indicate that UC‑MSCs alleviate STZ‑induced DR in rats by regulating angiogenesis and the inflammatory response at the molecular level. Thus, the findings of the present study may provide a theoretical basis for the application of MSCs in the treatment of DR.
-
FC/FACS
-
Rattus norvegicus (Rat)
-
Stem Cells and Developmental Biology
In PLoS One on 9 May 2009 by Pino, S. C., O'Sullivan-Murphy, B., et al.
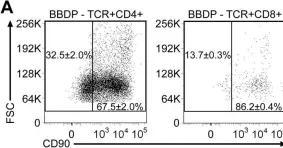
Fig.4.A

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1