Decellularized scaffolds are promising biomaterials for tissue and organ reconstruction; however, strategies to effectively suppress the host immune responses toward these implants, particularly those without chemical crosslinking, remain warranted. Administration of mesenchymal stem cells is effective against immune-mediated inflammatory disorders. Herein, we investigated the effect of isogeneic abdominal adipose-derived mesenchymal stem/stromal cells (ADMSCs) on xenogeneic biomaterial-induced immunoreactions. Peripheral bronchi from pigs, decellularized using a detergent enzymatic method, were engrafted onto tracheal defects of Brown Norway (BN) rats. BN rats were implanted with native pig bronchi (Xenograft group), decellularized pig bronchi (Decellularized Xenograft), or Decellularized Xenograft and ADMSCs (Decellularized Xenograft+ADMSC group). In the latter group, ADMSCs were injected intravenously immediately post implantation. Harvested graft implants were assessed histologically and immunohistochemically. We found that acute rejections were milder in the Decellularized Xenograft and Decellularized Xenograft+ADMSC groups than in the Xenograft group. Mild inflammatory cell infiltration and reduced collagen deposition were observed in the Decellularized Xenograft+ADMSC group. Additionally, ADMSC administration decreased CD8+ lymphocyte counts but increased CD163+ cell counts. In the Decellularized Xenograft+ADMSC group, serum levels of vascular endothelial growth factor and IL-10 were elevated and tissue deposition of IgM and IgG was low. The significant immunosuppressive effects of ADMSCs illustrate their potential use as immunosuppressive agents for xenogeneic biomaterial-based implants.
Product Citations: 29
In Organogenesis on 31 December 2023 by Hisanaga, M., Tsuchiya, T., et al.
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
-
Veterinary Research
In Cell Transplantation on 11 November 2023 by Tanoue, Y., Tsuchiya, T., et al.
Cell therapy using mesenchymal stromal cells (MSCs) is being studied for its immunosuppressive effects. In organ transplantation, the amount of MSCs that accumulate in transplanted organs and other organs may differ depending on administration timing, which may impact their immunosuppressive effects. In vitro, adipose-derived mesenchymal stem cells (ADMSCs) suppress lymphocyte activation under cell-to-cell contact conditions. However, in vivo, it is controversial whether ADMSCs are more effective in accumulating in transplanted organs or in secondary lymphoid organs. Herein, we aimed to investigate whether the timing of ADMSC administration affects its immunosuppression ability in a rat lung transplantation model. In the transplantation study, rats were intramuscularly administered half the usual dose of tacrolimus (0.5 mg/kg) every 24 h after lung transplantation. ADMSCs (1 × 106) were administered via the jugular vein before (PreTx) or after (PostTx) transplantation. Cell tracking using quantum dots was performed. ADMSCs accumulated predominantly in the lung and liver; fewer ADMSCs were distributed in the grafted lung in the PreTx group than in the PostTx group. The rejection rate was remarkably low in the ADMSC-administered groups, particularly in the PostTx group. Serum tumor necrosis factor-α (TNF-α), interferon-γ, and interleukin (IL)-6 levels showed a greater tendency to decrease in the PreTx group than in the PostTx group. The proportion of regulatory T cells in the grafted lung 10 days after transplantation was higher in the PostTx group than in the PreTx group. PostTx administration suppresses rejection better than PreTx administration, possibly due to regulatory T cell induction by ADMSCs accumulated in the transplanted lungs, suggesting a mechanism different from that in heart or kidney transplantation that PreTx administration is more effective than PostTx administration. These results could help establish cell therapy using MSCs in lung transplantation.
Parabiosis in Mice to Study Tissue Residency of Immune Cells.
In Current Protocols on 1 May 2022 by Wang, H., Gavil, N. V., et al.
Different populations of immune cells rely on their distinct migration patterns for immunosurveillance, immune regulation, tissue specific differentiation, and maturation. It is often important to clarify whether cells are recirculating or tissue resident, or whether tissue-specific cells are derived from blood-borne precursors or a tissue-resident population. Though migration or tissue residency of immune cells critically depends on the expression of different homing molecules (chemokine receptors, tissue retention molecules, etc.), characterization based solely on the expression of homing molecules may not faithfully reflect the migration patterns of immune cells. Therefore, a more reliable method to clarify migration patterns of immune cells is required. Parabiosis is a surgical connection of two mice resulting in a shared circulatory system, which allows reliable distinction of tissue-resident and circulating cells. Here, we describe a set of protocols for parabiosis, including technique details, pitfalls, and suggestions for optimization and troubleshooting. © 2022 Wiley Periodicals LLC. Basic Protocol 1: Preparation of mice for parabiosis surgery Basic Protocol 2: Parabiosis surgery Basic Protocol 3: Recovery and use of mice after parabiosis surgery Basic Protocol 4: Reversal of parabiotic surgery Basic Protocol 5: Analysis of parabionts.
© 2022 Wiley Periodicals LLC.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Methods and Protocols on 27 October 2021 by Bozhokin, M. S., Bozhkova, S. A., et al.
Damage to the hyaline layer of the articular surface is an urgent problem for millions of people around the world. At present, a large number of experimental methods are being developed to address this problem, including the transplantation of a cell-engineered construct (CEC) composed of a biodegradable scaffold with a premixed cell culture into the damaged area of the articular surface. However, current methods for analyzing the effectiveness of such CECs have significant limitations. This study aimed to compare the SEM technique, classical histology, and cryosectioning for the analysis of CECs transplanted to hyaline cartilage.
-
FC/FACS
-
Rattus norvegicus (Rat)
In Stem Cells and Development on 15 October 2020 by Wang, Z., Cui, M., et al.
Stem cell therapy provides an attractive solution for intervertebral disc (IVD) degeneration. However, the degenerative microenvironment, characterized by excessive mechanical loading and hypoxia, remains an obstacle for the long-lasting survival of exogenous transplanted stem cells. Whether and how bone marrow mesenchymal stem cells (BMSCs) adapt to the hostile microenvironment remain unclear. In this study, CoCl2 and mechanical compression were simultaneously used to simulate the hypoxic and overloaded microenvironment of IVDs in vitro. Compression had a proapoptotic effect through activation of the mitochondrial apoptotic pathway, while hypoxia exerted a prosurvival effect counteracting compression-induced apoptosis. Inhibiting the transcriptional activity of hypoxia inducible factor 1 subunit alpha (HIF-1α) by chetomin reversed the antiapoptotic effect of hypoxia. Furthermore, HIF-1α promoted dephosphorylation and activation of yes-associated protein (YAP) in hypoxic conditions. Conversely, both YAP inhibition and increased cell apoptosis were observed after inhibition through chetomin or YAP inhibitor verteporfin. Immunofluorescence staining and coimmunoprecipitation assays revealed that YAP could interact directly with HIF-1α and colocalize in the nucleus. Taken together, our results demonstrated that hypoxia protected BMSCs against compression-induced apoptosis in the degenerative disc microenvironment through activation of the HIF-1α/YAP signaling pathway. Thus, regulation of HIF-1α/YAP signaling might provide novel insights for promoting long-lasting BMSC survival and optimizing stem cell therapy for IVD degeneration.
-
FC/FACS
-
Stem Cells and Developmental Biology
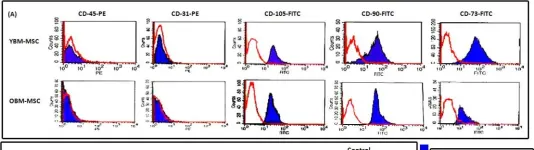
In BMC Cell Biol on 12 October 2011 by Asumda, F. Z. & Chase, P. B.
Fig.1.A

-
FC/FACS
-
Rattus norvegicus (Rat)
Collected and cropped from BMC Cell Biol by CiteAb, provided under a CC-BY license
Image 1 of 1