Rationale: It has been emergingly recognized that apoptosis generates plenty of heterogeneous apoptotic vesicles (apoVs), which play a pivotal role in the maintenance of organ and tissue homeostasis. However, it is unknown whether apoVs influence postnatal ovarian folliculogenesis. Methods: Apoptotic pathway deficient mice including Fas mutant (Fasmut ) and Fas ligand mutant (FasLmut ) mice were used with apoV replenishment to evaluate the biological function of apoVs during ovarian folliculogenesis. Ovarian function was characterized by morphological analysis, biochemical examination and cellular assays. Mechanistical studies were assessed by combinations of transcriptomic and proteomic analysis as well as molecular assays. CYP17A1-Cre; Axin1fl /fl mice was established to verify the role of WNT signaling during ovarian folliculogenesis. Polycystic ovarian syndrome (PCOS) mice and 15-month-old mice were used with apoV replenishment to further validate the therapeutic effects of apoVs based on WNT signaling regulation. Results: We show that systemic administration of mesenchymal stem cell (MSC)-derived apoptotic vesicles (MSC-apoVs) can ameliorate impaired ovarian folliculogenesis, PCOS phenotype, and reduced birth rate in Fasmut and FasLmut mice. Mechanistically, transcriptome analysis results revealed that MSC-apoVs downregulated a number of aberrant gene expression in Fasmut mice, which were enriched by kyoto encyclopedia of genes and genomes (KEGG) pathway analysis in WNT signaling and sex hormone biosynthesis. Furthermore, we found that apoptotic deficiency resulted in aberrant WNT/β-catenin activation in theca and mural granulosa cells, leading to responsive action of dickkopf1 (DKK1) in the cumulus cell and oocyte zone, which downregulated WNT/β-catenin expression in oocytes and, therefore, impaired ovarian folliculogenesis via NPPC/cGMP/PDE3A/cAMP cascade. When WNT/β-catenin was specially activated in theca cells of CYP17A1-Cre; Axin1fl /fl mice, the same ovarian impairment phenotypes observed in apoptosis-deficient mice were established, confirming that aberrant activation of WNT/β-catenin in theca cells caused the impairment of ovarian folliculogenesis. We firstly revealed that apoVs delivered WNT membrane receptor inhibitor protein RNF43 to ovarian theca cells to balance follicle homeostasis through vesicle-cell membrane integration. Systemically infused RNF43-apoVs down-regulated aberrantly activated WNT/β-catenin signaling in theca cells, contributing to ovarian functional maintenance. Since aging mice have down-regulated expression of WNT/β-catenin in oocytes, we used MSC-apoVs to treat 15-month-old mice and found that MSC-apoVs effectively ameliorated the ovarian function and fertility capacity of these aging mice through rescuing WNT/β-catenin expression in oocytes. Conclusion: Our studies reveal a previously unknown association between apoVs and ovarian folliculogenesis and suggest an apoV-based therapeutic approach to improve oocyte function and birth rates in PCOS and aging.
© The author(s).
Product Citations: 42
In Theranostics on 10 June 2024 by Fu, Y., Zhang, M., et al.
-
Stem Cells and Developmental Biology
In Scientific Reports on 14 May 2024 by Farouk, A. H., Aref, A., et al.
Due to vincristine sulfate's (VCR sulfate) toxicity and non-specific targeting, which might adversely damage healthy cells, its clinical application is restricted. In this study, we loaded VCR sulfate on exosomes generated from mesenchymal stem cells (MSCs) to enhance its targeted distribution. Exosomes are able to deliver molecules to specific cells and tissues and have therapeutic potential. In this study, we isolated exosomes from MSCs, and using probe-sonication approach loaded them with VCR sulfate. Using SRB assay, the cytotoxicity of VCR sulfate-Exo was assessed in T47D breast cancer cells, and the results were contrasted with those of free VCR sulfate. Then We labeled markers (CD44+/CD24-) in the cell line to assess the targeting effectiveness of VCR sulfate-Exo using flow cytometry. Our results showed that the cytotoxicity of VCR sulfate-Exo was nearly the same as that of VCR sulfate. Flow cytometry analysis revealed that VRC sulfate-Exo was more effectively targeted to MSCs than free VCR sulfate. Our study shows that loading VCR sulfate to MSCs-derived exosomes can improve their targeted delivery and lessen their side effects. Additional research is required to determine VCR sulfate-Exo's in vivo effectiveness and safety and improve the loading and delivery strategies.
© 2024. The Author(s).
-
Cancer Research
-
Stem Cells and Developmental Biology
Preprint on Research Square on 4 August 2023 by Farouk, A. H., Aref, A., et al.
Due to vincristine sulfate's (VCR sulfate) toxicity and non-specific targeting, which might adversely damage healthy cells, its clinical application is restricted. In this study, we loaded VCR sulfate on exosomes generated from mesenchymal stem cells (MSCs) to enhance its targeted distribution. Exosomes are able to deliver molecules to specific cells and tissues and have therapeutic potential. In this study we isolated exosomes from MSCs, and using probe-sonication approach loaded them with VCR sulfate. Using SRB assay, cytotoxicity of VCR sulfate-Exo was assessed in T47D breast cancer cells, and the results were contrasted with those of free VCR sulfate. Then We labelled markers (CD44+/CD24-) in the cell line to assess the targeting effectiveness of VCR sulfate-Exo using flow cytometry. Our results showed that the cytotoxicity of VCR sulfate-Exo was nearly the same as VCR sulfate. Analysis using flow cytometry revealed that VRC sulfate-Exo was more effectively targeted to MSCs than free VCR sulfate. Our study shows that loading VCR sulfate to MSCs derived exosomes can improve their targeted delivery and lessen their side effects. Additional research is required to determine the in vivo effectiveness and safety of VCR sulfate-Exo and to improve the loading and delivery strategies.
-
Cancer Research
-
Stem Cells and Developmental Biology
An IL-17-EGFR-TRAF4 axis contributes to the alleviation of lung inflammation in severe influenza.
In Communications Biology on 3 June 2023 by Dutta, A., Hung, C. Y., et al.
Excessive inflammation is a postulated cause of severe disease and death in respiratory virus infections. In response to severe influenza virus infection, adoptively transferred naïve hemagglutinin-specific CD4+ T cells from CD4+ TCR-transgenic 6.5 mice drive an IFN-γ-producing Th1 response in wild-type mice. It helps in virus clearance but also causes collateral damage and disease aggravation. The donor 6.5 mice have all the CD4+ T cells with TCR specificity toward influenza hemagglutinin. Still, the infected 6.5 mice do not suffer from robust inflammation and grave outcome. The initial Th1 response wanes with time, and a prominent Th17 response of recent thymic emigrants alleviates inflammation and bestows protection in 6.5 mice. Our results suggest that viral neuraminidase-activated TGF-β of the Th1 cells guides the Th17 evolution, and IL-17 signaling through the non-canonical IL-17 receptor EGFR activates the scaffold protein TRAF4 more than TRAF6 during alleviation of lung inflammation in severe influenza.
© 2023. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
GOT1 regulates CD8+ effector and memory T cell generation.
In Cell Reports on 31 January 2023 by Xu, W., Patel, C. H., et al.
T cell activation, proliferation, function, and differentiation are tightly linked to proper metabolic reprogramming and regulation. By using [U-13C]glucose tracing, we reveal a critical role for GOT1 in promoting CD8+ T cell effector differentiation and function. Mechanistically, GOT1 enhances proliferation by maintaining intracellular redox balance and serine-mediated purine nucleotide biosynthesis. Further, GOT1 promotes the glycolytic programming and cytotoxic function of cytotoxic T lymphocytes via posttranslational regulation of HIF protein, potentially by regulating the levels of α-ketoglutarate. Conversely, genetic deletion of GOT1 promotes the generation of memory CD8+ T cells.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
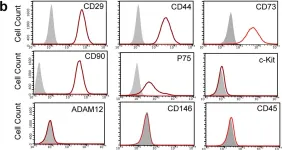
In Sci Rep on 10 January 2017 by Wang, D., Wang, A., et al.
Fig.3.B

-
FC/FACS
-
Rattus norvegicus (Rat)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 1