Passive immunotherapy is one of the most promising interventions for Alzheimer's disease (AD). However, almost all immune-modulating strategies fail in clinical trials with unclear causes although they attenuate neuropathology and cognitive deficits in AD animal models. Here, we showed that Aβ-targeting antibodies including their lgG1 and lgG4 subtypes induced microglial engulfment of neuronal synapses by activating CR3 or FcγRIIb via the complex of Aβ, antibody, and complement. Notably, anti-Aβ antibodies without Fc fragment, or with blockage of CR3 or FcγRIIb, did not exert these adverse effects. Consistently, Aβ-targeting antibodies, but not their Fab fragments, significantly induced acute microglial synapse removal and rapidly exacerbated cognitive deficits and neuroinflammation in APP/PS1 mice post-treatment, whereas the memory impairments in mice were gradually rescued thereafter. Since the recovery rate of synapses in humans is much lower than that in mice, our findings may clarify the variances in the preclinical and clinical studies assessing AD immunotherapies. Therefore, Aβ-targeting antibodies lack of Fc fragment, or with reduced Fc effector function, may not induce microglial synaptic pruning, providing a safer and more efficient therapeutic alternative for passive immunotherapy for AD.
© 2022. The Author(s).
Product Citations: 9
In Signal Transduction and Targeted Therapy on 25 January 2023 by Sun, X. Y., Yu, X. L., et al.
-
Rattus norvegicus (Rat)
-
Neuroscience
In Molecular Medicine on 3 December 2020 by Jo, B. G., Kim, S. H., et al.
Increasing number of studies provide evidence that the vagus nerve stimulation (VNS) dampens inflammation in peripheral visceral organs. However, the effects of afferent fibers of the vagus nerve (AFVN) on anti-inflammation have not been clearly defined. Here, we investigate whether AFVN are involved in VNS-mediated regulation of hepatic production of proinflammatory cytokines.
An animal model of hepatitis was generated by intraperitoneal (i.p.) injection of concanavalin A (ConA) into rats, and electrical stimulation was given to the hepatic branch of the vagus nerve. AFVN activity was regulated by administration of capsaicin (CAP) or AP-5/CNQX and the vagotomy at the hepatic branch of the vagus nerve (hVNX). mRNA and protein expression in target tissues was analyzed by RT-PCR, real-time PCR, western blotting and immunofluorescence staining. Hepatic immune cells were analyzed by flow cytometry.
TNF-α, IL-1β, and IL-6 mRNAs and proteins that were induced by ConA in the liver macrophages were significantly reduced by the electrical stimulation of the hepatic branch of the vagus nerve (hVNS). Alanine transaminase (ALT) and aspartate transaminase (AST) levels in serum and the number of hepatic CD4+ and CD8+ T cells were increased by ConA injection and downregulated by hVNS. CAP treatment deteriorated transient receptor potential vanilloid 1 (TRPV1)-positive neurons and increased caspase-3 signals in nodose ganglion (NG) neurons. Concomitantly, CAP suppressed choline acetyltransferase (ChAT) expression that was induced by hVNS in DMV neurons of ConA-injected animals. Furthermore, hVNS-mediated downregulation of TNF-α, IL-1β, and IL-6 expression was hampered by CAP treatment and similarly regulated by hVNX and AP-5/CNQX inhibition of vagal feedback loop pathway in the brainstem. hVNS elevated the levels of α7 nicotinic acetylcholine receptors (α7 nAChR) and phospho-STAT3 (Tyr705; pY-STAT3) in the liver, and inhibition of AFVN activity by CAP, AP-5/CNQX and hVNX or the pharmacological blockade of hepatic α7 nAChR decreased STAT3 phosphorylation.
Our data indicate that the activity of AFVN contributes to hepatic anti-inflammatory responses mediated by hVNS in ConA model of hepatitis in rats.
-
IHC-IF
-
ICC-IF
-
Rattus norvegicus (Rat)
-
Biochemistry and Molecular biology
-
Immunology and Microbiology
-
Neuroscience
Effect of pioglitazone on neuropathic pain and spinal expression of TLR-4 and cytokines.
In Experimental and Therapeutic Medicine on 1 October 2016 by Jia, H., Xu, S., et al.
The molecular mechanisms underlying neuropathic pain have yet to be elucidated. The present study aimed to examine the modulation of neuroimmune activation in the spinal cord by the synthetic peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist, pioglitazone (Pio), in a rat model of neuropathic pain induced by chronic constriction injury (CCI). Rats were randomly assigned into four groups: Sham surgery with vehicle, chronic constriction injury with vehicle or Pio (10 mg/kg), and chronic constriction injury with Pio and a PPAR-γ antagonist GW9662 (2 mg/kg). Pio or vehicle was administered 1 h prior to the surgery and continued daily until day 7 post-surgery. Paw pressure threshold was measured prior to surgery and on days 0, 1, 3 and 7 post-surgery. Microglia activation markers macrophage antigen complex-1, the mRNA expression levels of tumor necrosis factor α and interleukin-1β, and the mRNA expression levels of toll like receptor (TLR-4) in the lumbar spinal cord were determined. Administration of Pio resulted in the prominent attenuation of mechanical hyperalgesia. In addition, Pio was able to significantly inhibit neuroimmune activation characterized by glial activation, the production of cytokines and expression levels of TLR-4. Concurrent administration of a PPAR-γ antagonist, GW9662, reversed the effects of Pio. The antihyperalgesic effect of administration of Pio in rats receiving CCI may, in part, be attributed to the inhibition of neuroimmune activation associated with the sustaining of neuropathic pain.
-
IHC
-
Rattus norvegicus (Rat)
-
Neuroscience
In American Journal of Physiology - Heart and Circulatory Physiology on 1 March 2014 by Rodriguez-Menocal, L., Faridi, M. H., et al.
Aging has been associated with pathological vascular remodeling and increased neointimal hyperplasia. The understanding of how aging exacerbates this process is fundamental to prevent cardiovascular complications in the elderly. This study proposes a mechanism by which aging sustains leukocyte adhesion, vascular inflammation, and increased neointimal thickness after injury. The effect of aging on vascular remodeling was assessed in the rat balloon injury model using microarray analysis, immunohistochemistry, and LINCOplex assays. The injured arteries in aging rats developed thicker neointimas than those in younger animals, and this significantly correlated with a higher number of tissue macrophages and increased vascular IL-18. Indeed, IL-18 was 23-fold more abundant in the injured vasculature of aged animals compared with young rats, while circulating levels were similar in both groups of animals. The depletion of macrophages in aged rats with clodronate liposomes ameliorated vascular accumulation of IL-18 and significantly decreased neointimal formation. IL-18 was found to inhibit apoptosis of vascular smooth muscle cells (VSMC) and macrophages, thus favoring both the formation and inflammation of the neointima. In addition, injured arteries of aged rats accumulated 18-fold more fibrinogen-γ than those of young animals. Incubation of rat peritoneal macrophages with immobilized IL-18 increased leukocyte adhesion to fibrinogen and suggested a proinflammatory positive feedback loop among macrophages, VSMC, and the deposition of fibrinogen during neointimal hyperplasia. In conclusion, our data reveal that concentration changes in vascular cytokine and fibrinogen following injury in aging rats contribute to local inflammation and postinjury neointima formation.
-
Endocrinology and Physiology
-
Immunology and Microbiology
A CD28 superagonistic antibody elicits 2 functionally distinct waves of T cell activation in rats.
In The Journal of Clinical Investigation on 1 April 2008 by Müller, N., van den Brandt, J., et al.
Administration of the CD28 superagonistic antibody JJ316 is an efficient means to treat autoimmune diseases in rats, but the humanized antibody TGN1412 caused devastating side effects in healthy volunteers during a clinical trial. Here we show that JJ316 treatment of rats induced a dramatic redistribution of T lymphocytes from the periphery to the secondary lymphoid organs, resulting in severe T lymphopenia. Live imaging of secondary lymphoid organs revealed that JJ316 administration almost instantaneously (<2 minutes) arrested T cells in situ. This reduction in T cell motility was accompanied by profound cytoskeletal rearrangements and increased cell size. In addition, surface expression of lymphocyte function-associated antigen-1 was enhanced, endothelial differentiation sphingolipid G protein-coupled receptor 1 and L selectin levels were downregulated, and the cells lost their responsiveness to sphingosine 1-phosphate-directed migration. These proadhesive alterations were accompanied by signs of strong activation, including upregulation of CD25, CD69, CD134, and proinflammatory mediators. However, this did not lead to a cytokine storm similar to the clinical trial. While most of the early changes disappeared within 48 hours, we observed that CD4+CD25+FoxP3+ regulatory T cells experienced a second phase of activation, which resulted in massive cell enlargement, extensive polarization, and increased motility. These data suggest that CD28 superagonists elicit 2 qualitatively distinct waves of activation.
-
Immunology and Microbiology
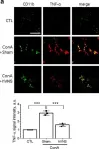
In Mol Med on 3 December 2020 by Jo, B. G., Kim, S. H., et al.
Fig.2.A

-
IHC-IF
-
Rattus norvegicus (Rat)
Collected and cropped from Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 2
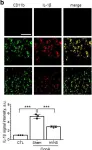
In Mol Med on 3 December 2020 by Jo, B. G., Kim, S. H., et al.
Fig.2.B

-
ICC-IF
-
Rattus norvegicus (Rat)
Collected and cropped from Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 2