Doxorubicin (DOX) is a potent chemotherapeutic agent and has toxic effects on various organs, including the liver. In the current study, we aimed to investigate the effects of bone-marrow-derived mesenchymal stem cell (BM-MSC) administration on DOX-induced hepatotoxicity in rats. 24 Wistar-albino rats were divided into three groups: Control, DOX, and DOX+MSC. DOX (20 mg/kg) was administered to the DOX group. In the DOX + MSC group, BM-MSCs (2 × 106 ) were given through the tail vein following DOX administration. DOX administration led to significant structural liver injury. Besides this, oxidative balance in the liver was impaired following DOX administration. DOX administration also led to an increase in apoptotic cell death in the liver. Structural and oxidative changes were significantly alleviated with the administration of BM-MSCs. Furthermore, BM-MSC administration suppressed excessive apoptotic cell death. Our findings revealed that BM-MSC administration may alleviate DOX-induced liver injury via improving the oxidative status and limiting apoptotic cell death in the liver tissue.
© 2022 Wiley Periodicals LLC.
Product Citations: 19
Effects of bone marrow-derived mesenchymal stem cells on doxorubicin-induced liver injury in rats.
In Journal of Biochemical and Molecular Toxicology on 1 April 2022 by Celik Samanci, T., Gökçimen, A., et al.
-
FC/FACS
-
Rattus norvegicus (Rat)
-
Biochemistry and Molecular biology
-
Stem Cells and Developmental Biology
Neuron-Derived Extracellular Vesicles Modulate Microglia Activation and Function.
In Biology on 22 September 2021 by Peng, H., Harvey, B. T., et al.
Microglia act as the immune cells of the central nervous system (CNS). They play an important role in maintaining brain homeostasis but also in mediating neuroimmune responses to insult. The interactions between neurons and microglia represent a key process for neuroimmune regulation and subsequent effects on CNS integrity. However, the molecular mechanisms of neuron-glia communication in regulating microglia function are not fully understood. One recently described means of this intercellular communication is via nano-sized extracellular vesicles (EVs) that transfer a large diversity of molecules between neurons and microglia, such as proteins, lipids, and nucleic acids. To determine the effects of neuron-derived EVs (NDEVs) on microglia, NDEVs were isolated from the culture supernatant of rat cortical neurons. When NDEVs were added to primary cultured rat microglia, we found significantly improved microglia viability via inhibition of apoptosis. Additionally, application of NDEVs to cultured microglia also inhibited the expression of activation surface markers on microglia. Furthermore, NDEVs reduced the LPS-induced proinflammatory response in microglia according to reduced gene expression of proinflammatory cytokines (TNF-α, IL-6, MCP-1) and iNOS, but increased expression of the anti-inflammatory cytokine, IL-10. These findings support that neurons critically regulate microglia activity and control inflammation via EV-mediated neuron-glia communication. (Supported by R21AA025563 and R01AA025591).
-
FC/FACS
-
Rattus norvegicus (Rat)
-
Neuroscience
Peptide 11R‑VIVIT promotes fracture healing in osteoporotic rats.
In International Journal of Molecular Medicine on 1 August 2021 by Hou, C., Wang, X., et al.
Osteoporotic fracture healing is a complex clinical issue. The present study was conducted to investigate the repair properties of 11R‑VIVIT on osteoporotic fractures and to examine the potential effects of 11R‑VIVIT on osteoporotic bone marrow‑derived mesenchymal stem cells (BMSCs), A rat model of osteoporotic femoral fracture was established, and the effects of the daily local injection of 11R‑VIVIT or saline on fracture repairing were evaluated by micro‑CT scans and H&E staining. Moreover, BMSCs from osteoporotic rats were treated with 11R‑VIVIT, and the osteogenic and adipogenic differentiation of BMSCs was evaluated. The results revealed that 11R‑VIVIT promoted bone formation and increased fracture healing. In addition, 11R‑VIVIT promoted the differentiation of osteoporotic BMSCs into osteoblasts rather than adipocytes. Furthermore, mechanistic analysis revealed that 11R‑VIVIT promoted autophagy by blocking the protein kinase B (AKT)/nuclear factor of activated T‑cells (NFATc1) signaling pathway. Consistently, the activation and inhibition of autophagy using rapamycin and LY294002 confirmed the regulatory effects of 11R‑VIVIT on autophagy. On the whole, the findings of the present study demonstrate that 11R‑VIVIT promotes fracture healing in osteoporotic rats and enhances the osteogenic differentiation of osteoporotic BMSCs by dysregulating the AKT/NFATc1 signaling pathway.
-
FC/FACS
-
Rattus norvegicus (Rat)
A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment.
In The Journal of Experimental Medicine on 5 April 2021 by Balestrini, A., Joseph, V., et al.
Despite the development of effective therapies, a substantial proportion of asthmatics continue to have uncontrolled symptoms, airflow limitation, and exacerbations. Transient receptor potential cation channel member A1 (TRPA1) agonists are elevated in human asthmatic airways, and in rodents, TRPA1 is involved in the induction of airway inflammation and hyperreactivity. Here, the discovery and early clinical development of GDC-0334, a highly potent, selective, and orally bioavailable TRPA1 antagonist, is described. GDC-0334 inhibited TRPA1 function on airway smooth muscle and sensory neurons, decreasing edema, dermal blood flow (DBF), cough, and allergic airway inflammation in several preclinical species. In a healthy volunteer Phase 1 study, treatment with GDC-0334 reduced TRPA1 agonist-induced DBF, pain, and itch, demonstrating GDC-0334 target engagement in humans. These data provide therapeutic rationale for evaluating TRPA1 inhibition as a clinical therapy for asthma.
© 2021 Genentech.
-
FC/FACS
-
Rattus norvegicus (Rat)
-
Immunology and Microbiology
Protective effect and mechanism of mesenchymal stem cells on heat stroke induced intestinal injury.
In Experimental and Therapeutic Medicine on 1 October 2020 by Wang, L., Deng, Z., et al.
Heat stroke (HS) is considered to be a severe systemic inflammatory reaction disease that is caused by high fever. The mortality of HS is high worldwide due to the lack of effective treatments. Presently, mesenchymal stem cells (MSCs) have been demonstrated to serve roles in inflammation and immune regulation. Therefore, the current study aimed to investigate the protective effect and mechanism of MSCs against the HS-induced inflammatory response and organ dysfunction. A rat model of HS was induced by a high-temperature environment and treated with MSCs via tail veins. The levels of molecular markers of organ function, inflammatory factors and chemokines were examined at days 1, 7, 14 and 28. Histological staining was performed on the intestines of rats and control groups, and the Chiu's scores of the two groups were compared. The results revealed that MSCs injection significantly reduced the mortality and inhibited the circulatory inflammatory response. Additionally, main organ function, such as in the liver and kidney, were significantly improved following MSCs infusion in HS rats. Furthermore, MSCs treatment significantly improved edema, necrosis and villus exfoliation of intestinal mucosa, and reduced the inflammatory response of intestinal tissue. These results indicated that MSC infusion had therapeutic effects on HS of rats by regulating the circulatory and intestinal inflammatory response. Moreover, MSCs may be able to protect organ function and promote tissue repair in HS. The results of the current study indicated that MSCs may be used as a potential method to treat HS and the resulting organ dysfunction.
Copyright: © Wang et al.
-
FC/FACS
-
Rattus norvegicus (Rat)
-
Cardiovascular biology
-
Stem Cells and Developmental Biology
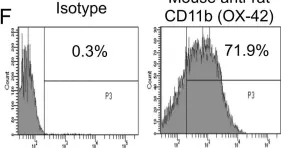
In PLoS One on 3 November 2012 by Signarovitz, A. L., Ray, H. J., et al.
Fig.1.F

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 5
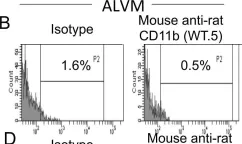
In PLoS One on 3 November 2012 by Signarovitz, A. L., Ray, H. J., et al.
Fig.1.B

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 5
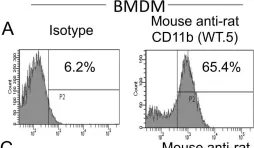
In PLoS One on 3 November 2012 by Signarovitz, A. L., Ray, H. J., et al.
Fig.1.A

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 5
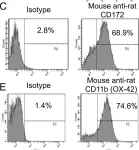
In PLoS One on 3 November 2012 by Signarovitz, A. L., Ray, H. J., et al.
Fig.1.C

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 5
In PLoS One on 3 November 2012 by Signarovitz, A. L., Ray, H. J., et al.
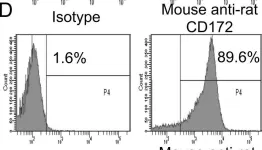
Fig.1.D

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 5