Despite the potential, bone marrow-derived mesenchymal stem cells (BMSCs) show limitations for beta (ß)-cell replacement therapy due to inefficient methods to deliver BMSCs into pancreatic lineage. In this study, we report TGF-ß family member protein, Activin-a potential to stimulate efficient pancreatic migration, enhanced homing and accelerated ß-cell differentiation.
Lineage tracing of permanent green fluorescent protein (GFP)- tagged donor murine BMSCs transplanted either alone or in combination with Activin-a in diabetic mice displayed potential ß-cell regeneration and reversed diabetes.
Pancreatic histology of Activin-a treated recipient mice reflected high GFP+BMSC infiltration into damaged pancreas with normalized fasting blood glucose and elevated serum insulin. Whole pancreas FACS profiling of GFP+ cells displayed significant homing of GFP+BMSC with Activin-a treatment (6%) compared to BMSCs alone transplanted controls (0.5%). Within islets, approximately 5% GFP+ cells attain ß-cell signature (GFP+ Ins+) with Activin-a treatment versus controls. Further, double immunostaining for mesenchymal stem cell markers CD44+/GFP+ in infiltrated GFP+BMSC deciphers substantial endocrine reprogramming and ß-cell differentiation (6.4% Ins+/GFP+) within 15 days.
Our investigation thus presents a novel pharmacological approach for stimulating direct migration and homing of therapeutic BMSCs that re-validates BMSC potential for autologous stem cell transplantation therapy in diabetes.
Product Citations: 6
In Stem Cell Research & Therapy on 30 July 2020 by Dadheech, N., Srivastava, A., et al.
-
Mus musculus (House mouse)
-
Stem Cells and Developmental Biology
Brief exposure to hyperglycemia activates dendritic cells in vitro and in vivo.
In Journal of Cellular Physiology on 1 June 2020 by Thomas, A. M., Dong, Y., et al.
Dendritic cells are key players in regulating immunity. These cells both activate and inhibit the immune response depending on their cellular environment. Their response to hyperglycemia, a condition common amongst diabetics wherein glucose is abnormally elevated, remains to be elucidated. In this study, the phenotype and immune response of dendritic cells exposed to hyperglycemia were characterized in vitro and in vivo using the streptozotocin-induced diabetes model. Dendritic cells were shown to be sensitive to hyperglycemia both during and after differentiation from bone marrow precursor cells. Dendritic cell behavior under hyperglycemic conditions was found to vary by phenotype, among which, tolerogenic dendritic cells were particularly sensitive. Expression of the costimulatory molecule CD86 was found to reliably increase when dendritic cells were exposed to hyperglycemia. Additionally, hydrogel-based delivery of the anti-inflammatory molecule interleukin-10 was shown to partially inhibit these effects in vivo.
© 2019 Wiley Periodicals, Inc.
-
Endocrinology and Physiology
-
Immunology and Microbiology
In eLife on 27 January 2016 by Theodosiou, M., Widmaier, M., et al.
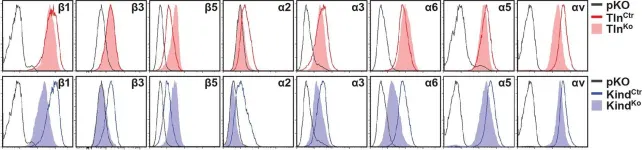
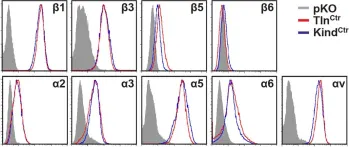
Integrins require an activation step prior to ligand binding and signaling. How talin and kindlin contribute to these events in non-hematopoietic cells is poorly understood. Here we report that fibroblasts lacking either talin or kindlin failed to activate β1 integrins, adhere to fibronectin (FN) or maintain their integrins in a high affinity conformation induced by Mn(2+). Despite compromised integrin activation and adhesion, Mn(2+) enabled talin- but not kindlin-deficient cells to initiate spreading on FN. This isotropic spreading was induced by the ability of kindlin to directly bind paxillin, which in turn bound focal adhesion kinase (FAK) resulting in FAK activation and the formation of lamellipodia. Our findings show that talin and kindlin cooperatively activate integrins leading to FN binding and adhesion, and that kindlin subsequently assembles an essential signaling node at newly formed adhesion sites in a talin-independent manner.
-
IF
-
FC/FACS
-
Mus musculus (House mouse)
Rac1 is crucial for hair follicle integrity but is not essential for maintenance of the epidermis.
In Molecular and Cellular Biology on 1 September 2006 by Chrostek, A., Wu, X., et al.
Rac1 is a small GTPase that regulates the actin cytoskeleton but also other cellular processes. To investigate the function of Rac1 in skin, we generated mice with a keratinocyte-restricted deletion of the rac1 gene. Rac1-deficient mice lost nearly all of their hair within a few weeks after birth. The nonpermanent part of mutant hair follicles developed constrictions; lost expression of hair follicle-specific keratins, E-cadherin, and alpha6 integrin; and was eventually removed by macrophages. The permanent part of hair follicles and the sebaceous glands were maintained, but no regrowth of full-length hair follicles was observed. In the skin of mutant mice, epidermal keratinocytes showed normal differentiation, proliferation, cell-cell contacts, and basement membrane deposition, demonstrating no obvious defects of Rac1-deficient epidermis in vivo. In vitro, Rac1-null keratinocytes displayed a strong spreading defect and slightly impaired adhesion. These data show that Rac1 plays an important role in sustaining the integrity of the lower part of hair follicles but not in maintenance of the epidermis.
-
Cell Biology
DX5/CD49b-positive T cells are not synonymous with CD1d-dependent NKT cells.
In The Journal of Immunology on 1 October 2005 by Pellicci, D. G., Hammond, K. J., et al.
NKT cells are typically defined as CD1d-dependent T cells that carry an invariant TCR alpha-chain and produce high levels of cytokines. Traditionally, these cells were defined as NK1.1+ T cells, although only a few mouse strains express the NK1.1 molecule. A popular alternative marker for NKT cells has been DX5, an Ab that detects the CD49b integrin, expressed by most NK cells and a subset of T cells that resemble NKT cells. Interpretation of studies using DX5 as an NKT cell marker depends on how well DX5 defines NKT cells. Using a range of DX5 and other anti-CD49b Abs, we reveal major differences in reactivity depending on which Ab and which fluorochrome are used. The brightest, PE-conjugated reagents revealed that while most CD1d-dependent NKT cells expressed CD49b, they represented only a minority of CD49b+ T cells. Furthermore, CD49b+ T cell numbers were near normal in CD1d-/- mice that are completely deficient for NKT cells. CD1d tetramer- CD49b+ T cells differ from NKT cells by their activation and memory marker expression, tissue distribution, and CD4/CD8 coreceptor profile. Interestingly, both NKT cells and CD1d tetramer- CD49b+ T cells produce cytokines, but the latter are clearly biased toward Th1-type cytokines, in contrast to NKT cells that produce both Th1 and Th2 cytokines. Finally, we demonstrate that expression of CD49b by NKT cells does not dramatically alter with age, contrasting with earlier reports proposing DX5 as a maturation marker for NKT cells. In summary, our data demonstrate that DX5/CD49b is a poor marker for identifying CD1d-dependent NKT cells.
-
Immunology and Microbiology
In Elife on 27 January 2016 by Theodosiou, M., Widmaier, M., et al.
Fig.2.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 2
In Elife on 27 January 2016 by Theodosiou, M., Widmaier, M., et al.
Fig.1.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 2